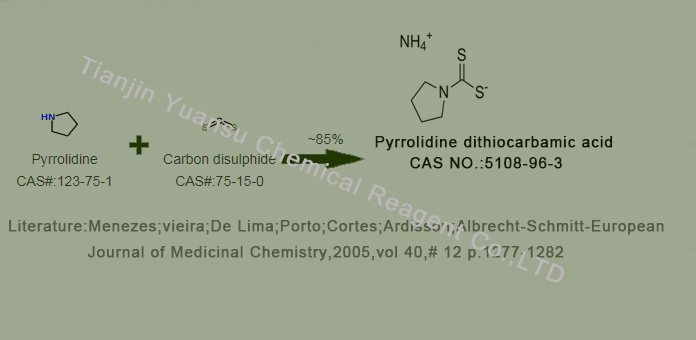
Pyrrolidine dithiocarbamic acid
What is Pyrrolidine dithiocarbamic acid, cas no:5108-96-3 ,a producer telling you the result.
CAS NO.5108-96-3
If you need the products .Please send your inquiry to us through e-mail: sales@yuansu-reagent.com
To begin with, let us tell you what is the basic information ofPyrrolidine dithiocarbamic acid ?
|
Molecular Formula |
C5H12N2S2 |
Molecular Weight |
164.292 |
|
Density |
1.264g/cm3 |
||
|
Flash Point |
74.6ºC |
Melting Point |
153-155 °C(lit.) |
Like many stuff, it has many synonyms as follows
|
MFCD00012720 |
|
Pyrrolindinedithiocarbamate ammonium |
|
1-Pyrrolidinecarbodithioic acid ammoniate (1:1) |
|
Ammonium Pyrrolidine-N-dithiocarbamate |
|
Pyrrolidine-N-dithiocarbamic Acid Ammonium Salt |
|
Ammonium 1-pyrrolidinedithiocarboxylate |
|
Ammonium pyrrolidinecarbodithioate |
|
APCD |
|
PDTC,NH4 |
|
PYRROLIDINEDITHIOCARBAMATE |
|
APDTC |
|
1-PYRROLIDINECARBODITHIOIC ACID AMMONIUM SALT |
|
QX 314 chloride |
|
Ammonium pyrrolidine |
|
1-Pyrrolidinecarbodithioic acid, ammonuim salt |
|
Ammonium pyrrolidine dithiocarbamate |
|
APDC |
|
Ammonium 1-Pyrrolidinecarbodithioate |
|
1-PYRROLIDINECARBODITHIOIC ACID |
|
Pyrrolidinedithiocarbamate ammonium |
|
APDC AMMONIUM SALT |
|
Ammonium 1-pyrrolidinedithiocarbamate |
|
Ammoniumpyrrolidinedithiocarb |
|
Ammonium pyrrolidine-1-carbodithioate |
|
1-Pyrrolidinecarbodithioic acid, ammonium salt |
|
1-Pyrrolidinecarbodithioic acid ammonium salt,APDC,Ammonium pyrrolidinecarbodithioate |
|
Ammonium pyrrolidinedithiocarbamate |
|
Pyrrolidinedithiocarbamate (ammonium)
|
First, the chemical is very special, some technical indexes as below
If you need the products .Please send your inquiry to us through e-mail: sales@yuansu-reagent.com
Density 1.117 (estimate)
Melting point 153-155 ° C (lit.)
Boiling point 329.4 ° C at 760 mmHg
Flash point 153 ° C
Water solubility 50 g/L (20 º C)
Steam pressure 0.000178mmHg at 25 ° C
Solubility 190g/l
Refractive index 1.5300 (estimate)
PH value 6-7 (50g/l, H2O, 20 ℃)
Storage conditions Store at+2 ° C to+8 ° C
Stability and stability. Incompatible with strong oxidants.
Sensitivity Hygroscopic
Second, the Synthetic Route we will recommend is the most important for your reference?
First, synthesis line ofPyrrolidine dithiocarbamic acid CAS NO.5108-96-3 as follows
Manufacturing method:
1. Synthesis of pyrrolidine: Acetone reacts with sulfur and ammonium cyanate in ethanol solvent to produce pyrrolidine.
2. Synthesis of ammonium pyrrolidine dithiocarbamate: Pyrrolidine is reacted with thioformic acid to obtain ammonium
pyrrolidine dithiocarbamate.
Third, what is the usage of Pyrrolidine dithiocarbamic acid CAS NO.5108-96-3 ? pleas see below
If you need the products .Please send your inquiry to us through e-mail:sales@yuansu-reagent.com
The uses of ammonium pyrrolidine dithiocarbamate are as follows:
Main Usage:
Used for the synthesis of Dithiobis(pyrrolizinomethanethione) Cas no. 496-08-2
Besides Safety Information ofPyrrolidine dithiocarbamic acid CAS NO.5108-96-3 is also important when handling it
|
Hazard Codes |
Xi |
|
WGK Germany |
3 |
|
H.S.Code: |
2933990090 |
|
TSCA |
Yes |
|
HazardClass |
IRRITANT |
What is the appearance of Pyrrolidine dithiocarbamic acid CAS NO.5108-96-3 ? Please see the picture ofPyrrolidine dithiocarbamic acid CAS NO.5108-96-3 , below
If you need the products .Please send your inquiry to us through e-mail: sales@yuansu-reagent.com
Specification ofPyrrolidine dithiocarbamic acid CAS NO.5108-96-3 , is below
Apperance: White crystalline powder, soluble in water and some organic solvents
Assay: 99 % min by HPLC
IR identity: conform
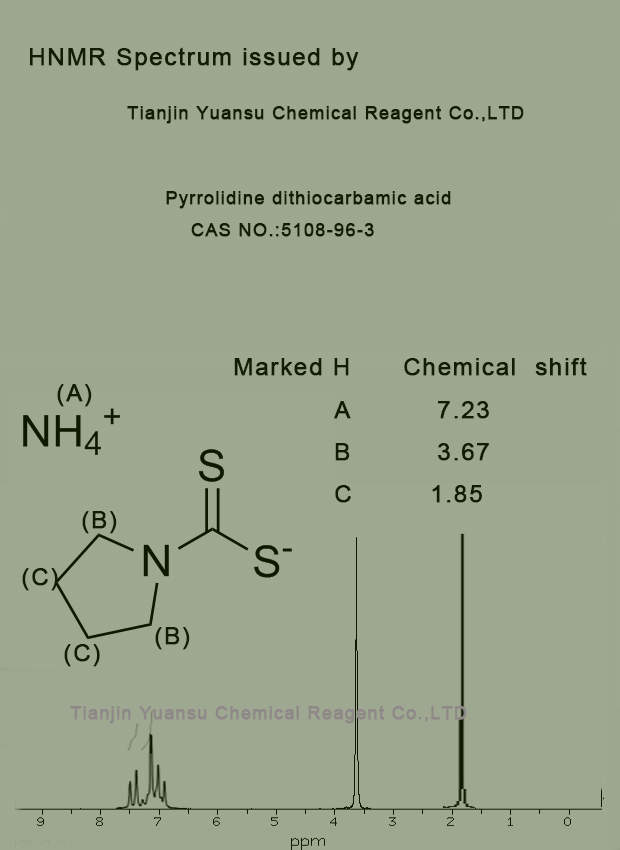
H-NMR Spectrum picture of Pyrrolidine dithiocarbamic acid CAS NO.5108-96-3 is as follows,
IR Spectrum picture of Pyrrolidine dithiocarbamic acid CAS NO.5108-96-3 is as follows,
Reference of Article cited for your reference below,
(1)
Dynamics of manganese, cadmium, and lead in experimental power plant ponds
Publication Date: 1979
Publication Name: Hydrobiologia
(2)
Recent Developments in Atomic Absorption and Flame Emission Spectroscopy
Publication Date: 1968
Publication Name: Developments in Applied Spectroscopy
(3)
Extraction techniques in speciation analysis of environmental samples
Publication Date: 1995
Publication Name: Fresenius' Journal of Analytical Chemistry