Tetrabutylammonium perchlorate
What is Tetrabutylammonium perchlorate, cas no:1923-70-2,a producer telling you the result.
CAS NO.1923-70-2
If you need the products .Please send your inquiry to us through e-mail: sales@yuansu-reagent.com
To begin with, let us tell you what is the basic information ofTetrabutylammonium perchlorate ?
|
CAS Number |
Molecular Weight |
341.914 |
|
|
Molecular Formula |
C16H36ClNO4 |
Melting Point |
211-215 °C |
Like many stuff, it has many synonyms as follows
|
MFCD00038722 |
|
N,N,N-Tributyl-1-butanaminium perchlorate |
|
tetra-n-butylammonium perchlorate |
|
Tetrabutylammonium Perchlorate |
|
tetrabutylammoniumperchlorate |
|
tetra(n-butyl)ammonium perchlorate |
|
Bu4N(1+)*ClO4(1-) |
|
tetrabutylazanium perchlorate |
|
n,n,n-tributylbutan-1-aminium perchlorate |
|
Tetrabutyl ammonium perchlorate |
|
Tetrabutylammonium Perchlorat |
First, the chemical is very special, some technical indexes as below
If you need the products .Please send your inquiry to us through e-mail: sales@yuansu-reagent.com
Physical property data
1. Appearance: Crystalline
2. Density (g/mL, 25 º C): undetermined
3. Relative vapor density (g/mL, air=1): not determined
4. Melting point (º C): 212
5. Boiling point (º C): undetermined
6. Boiling point (º C, 1mmHg): undetermined
7. Refractive index: undetermined
8. Flash point (° C): undetermined
9. Specific rotation (º F): not determined
10. Spontaneous combustion point or ignition temperature (º C): not determined
11. Vapor pressure (kPa, 25 º C): not determined
12. Saturated vapor pressure (kPa, 110 º C): not determined
13. Combustion heat (KJ/mol): undetermined
14. Critical temperature (º C): not determined
15. Critical pressure (KPa): undetermined
16. Logarithmic value of oil-water (octanol/water) partition coefficient: undetermined
17. Explosion upper limit (%, V/V): undetermined
18. Lower explosive limit (%, V/V): not determined
19. Solubility: Able to dissolve in water
toxicology data
Acute toxicity:
Main irritant effects:
On the skin: irritates the skin and mucous membranes.
Above the eyes: the impact of stimulation.
Sensitization effect: There is no known sensitization effect.
Ecological data
It is slightly harmful to water. Do not let undiluted or large amounts of products come into contact with groundwater, waterways, or sewage systems. Do not discharge materials into the surrounding environment without government permission.
Molecular structure data
None
Calculate chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 4
4. Number of rotatable chemical bonds: 12
5. Number of tautomers: None
6. Topological molecule polarity surface area 74.3
7. Number of heavy atoms: 22
8. Surface charge: 0
9. Complexity: 212
10. Number of isotopic atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 2
Nature and stability
If used and stored according to specifications, it will not decompose, there are no known hazardous reactions, and oxidation should be avoided
Storage
Keep the storage container sealed and stored in a cool, dry place, ensuring good ventilation or exhaust in the workspace
Second, the Synthetic Route we will recommend is the most important for your reference?
First, synthesis line ofTetrabutylammonium perchlorate CAS NO.1923-70-2 as follows
Synthetic method
There are various methods for preparing tetra-n-butylammonium perchlorate, and the common method is to obtain it by reacting n-butylamine with perchloric acid. The reaction conditions generally require lower temperatures and suitable solvents.
Third, what is the usage of Tetrabutylammonium perchlorate CAS NO.1923-70-2 ? pleas see below
If you need the products .Please send your inquiry to us through e-mail:sales@yuansu-reagent.com
Usage:
Used as a supporting electrolyte
-Tetra-n-butylammonium perchlorate is commonly used as an ionic liquid catalyst in organic synthesis, with high selectivity and catalytic activity.
-It can also be used as an electrochemical research reagent, such as in the application of lithium-ion batteries.
-Its good conductivity can also be used to prepare conductive polymer materials and electrolytes.
-Tetra-n-butylammonium perchlorate can also be used as an auxiliary propellant for rocket fuel and explosive
Main usages:
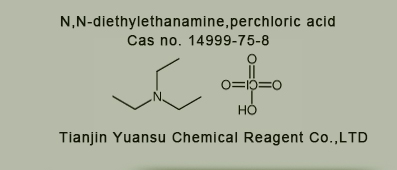
Used for the synthesis of N,N-diethylethanamine,perchloric acid Cas no. 14999-75-8
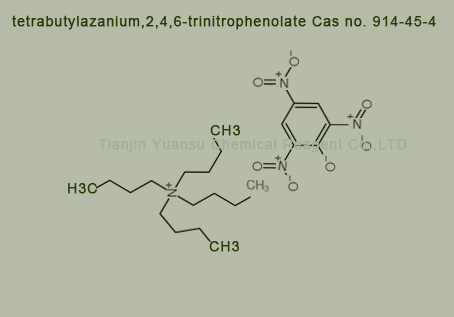
Used for the synthesis of tetrabutylazanium,2,4,6-trinitrophenolate Cas no. 914-45-4
Used for the synthesis of triphenyl(triphenylgermyloxy)germane Cas no. 2181-40-0
Besides Safety Information ofTetrabutylammonium perchlorate CAS NO.1923-70-2 is also important when handling it
|
Hazard Codes |
Xi |
|
WGK Germany |
3 |
|
H.S.Code: |
2923 9000.90 |
|
TSCA |
Yes |
|
HazardClass |
IRRITANT |
What is the appearance of Tetrabutylammonium perchlorate CAS NO.1923-70-2? Please see the picture ofTetrabutylammonium perchlorate CAS NO.1923-70-2, below
If you need the products .Please send your inquiry to us through e-mail: sales@yuansu-reagent.com
Specification of Tetrabutylammonium perchlorate CAS NO.1923-70-2, is below
Apperance: White crystalline solid, soluble in water and organic solvents
Assay: 99 min by GC
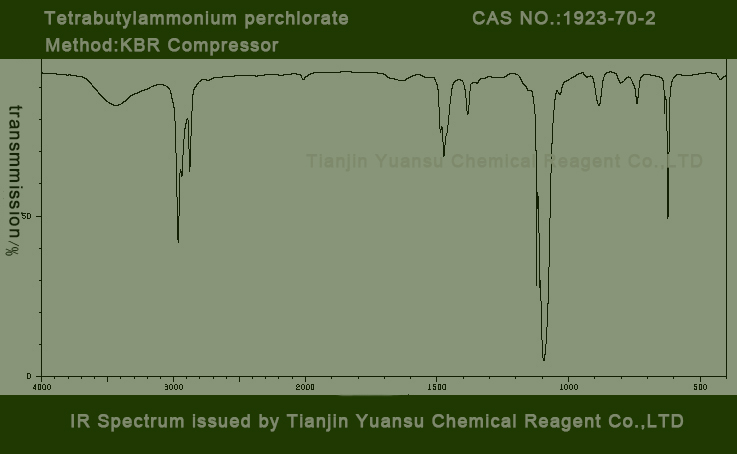
IR identity: conform
IR picture of Tetrabutylammonium perchlorate CAS NO.1923-70-2 is as follows,
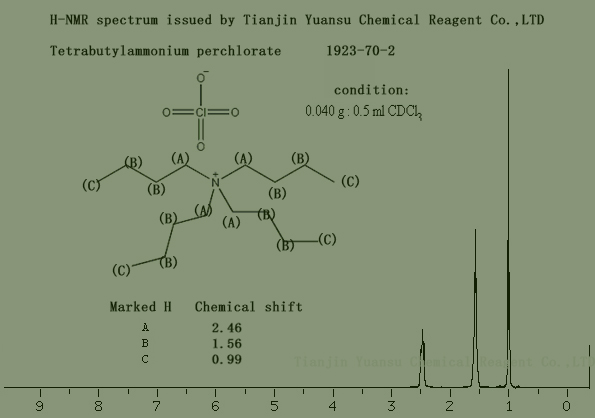
H-NMR Spectrum picture of Tetrabutylammonium perchlorate CAS NO.1923-70-2 is as follows,
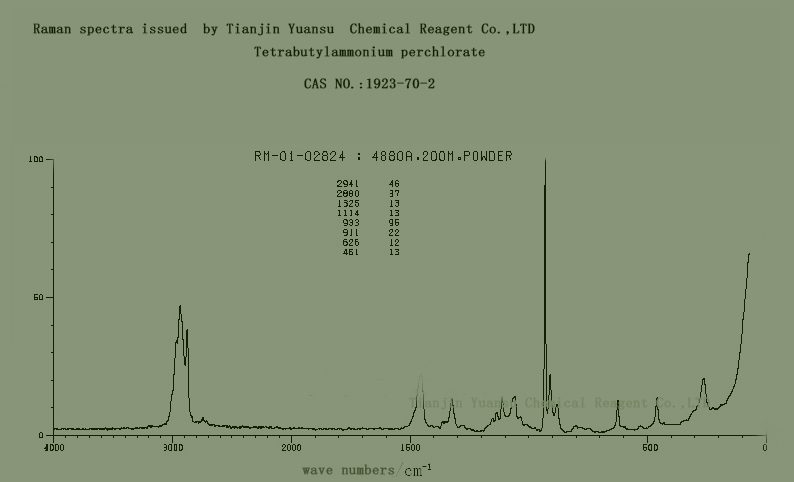
Raman spectra of Tetrabutylammonium perchlorate CAS NO.1923-70-2 is as follows
Reference of Article cited for your reference below,
(1)
Non-Fluorine Based Bulk Solution Techniques to Grow Superconducting YBa2Cu3O7−δ Films
Publication Date: 2005
Publication Name: Second-Generation HTS Conductors
(2)
Electrochemical Properties of Nanoparticle Assemblies
Publication Date: 2004
Publication Name: Self-Assembled Nanostructures
(3)
4.2.1.2.2.2.2 Neutral radicals
Publication Date: 1995
Publication Name: Carbon-Centered Radicals II