Bis(2-chloroethyl)amine hydrochloride
What is Bis(2-chloroethyl)amine hydrochloride, cas no:821-48-7,a producer telling you the result.
CAS NO.821-48-7
If you need the products .Please send your inquiry to us through e-mail: sales@yuansu-reagent.com
To begin with, let us tell you what is the basic information of Bis(2-chloroethyl)amine hydrochloride ?
|
Molecular Formula |
C4H10Cl3N |
Molecular Weight |
178.49 |
|
Density |
1.125 g/cm3 |
Boiling Point |
204.2ºC at 760 mmHg |
|
Flash Point |
77.3ºC |
Melting Point |
212-214 °C(lit.) |
Like many stuff, it has many synonyms as follows
|
2-Chloro-N-(2-chloroethyl)ethanamine hydrochloride (1:1) |
|
Bis(2-chloroethyl)amine Hydrochloride |
|
2-Chlor-N-(2-chlorethyl)ethanaminhydrochlorid |
|
Ethanamine, 2-chloro-N- (2-chloroethyl)-, hydrochloride |
|
Bis(β-chloroethyl)amine hydrochloride |
|
Ethanamine, 2-chloro-N-(2-chloroethyl)-, hydrochloride (1:1) |
|
Bis(2-Chloroethyl)Amine HCL |
First, the chemical is very special, some technical indexes as below
If you need the products .Please send your inquiry to us through e-mail: sales@yuansu-reagent.com
Physical property data
1. Appearance: Crystalline.
2. Density (g/mL, 25/4 ℃): not determined
3. Relative vapor density (g/mL, air=1): not determined
4. Melting point (º C): 212-214
5. Boiling point (º C, atmospheric pressure): undetermined
6. Boiling point (º C, 5.2kPa): undetermined
7. Refractive index: undetermined
8. Flash point (º C): undetermined
9. Specific rotation (º): not determined
10. Spontaneous combustion point or ignition temperature (º C): not determined
11. Vapor pressure (kPa, 25 º C): not determined
12. Saturated vapor pressure (kPa, 60 º C): not determined
13. Combustion heat (KJ/mol): undetermined
14. Critical temperature (º C): not determined
15. Critical pressure (KPa): undetermined
16. Logarithmic value of oil-water (octanol/water) partition coefficient: undetermined
17. Explosion upper limit (%, V/V): undetermined
18. Lower explosive limit (%, V/V): not determined
19. Solubility: undetermined
toxicology data
Acute toxicity: intraperitoneal LD50 in rats: 100mg/kg, inhalation LD50 in mice: 1000mg/m3/10M
Ecological data
Slightly harmful to water, do not allow undiluted or large amounts of products to come into contact with groundwater, waterways, or sewage systems. Do not discharge materials into the surrounding environment without government permission
Molecular structure data
None
Calculate chemical data
1. Number of hydrogen bond donors: 2
2. Number of hydrogen bond acceptors: 1
3. Number of rotatable chemical bonds: 4
4. Topological Molecular Polarity Surface Area (TPSA): 12
5. Number of heavy atoms: 8
6. Surface charge: 0
7. Complexity: 28.9
8. Number of isotopic atoms: 0
9. Determine the number of atomic stereocenters: 0
10. Uncertain number of atomic stereocenters: 0
11. Determine the number of chemical bond stereocenters: 0
12. Uncertain number of chemical bond stereocenters: 0
13. Number of covalent bond units: 2
Nature and stability
This product should be sealed and stored away from light.
Stable at room temperature and pressure, avoiding contact with oxides and moisture
Storage
Keep the container sealed and store in a cool, dry place
Second, the Synthetic Route we will recommend is the most important for your reference?
First, synthesis line ofBis(2-chloroethyl)amine hydrochloride CAS NO.821-48-7 as follows
Synthetic method
Obtained by the reaction of diethanolamine and thionyl chloride. Dissolve thionyl chloride in chloroform and add a mixture of diethanolamine and chloroform dropwise below 0 ℃. Control the droplet acceleration to maintain the temperature at -4-6 ℃. After adding, keep warm and stir for 30 minutes. Stir at room temperature for another hour, heat to 60-65 ℃, wait for all crystals to precipitate, add anhydrous ethanol, and heat to reflux to dissolve all. Pour out while hot, cool naturally, and precipitate crystals. Filter the next day and wash twice with chloroform or anhydrous ethanol. Dry to obtain the finished product, with a yield of 76%
Third, what is the usage of Bis(2-chloroethyl)amine hydrochloride CAS NO.821-48-7 ? pleas see below
If you need the products .Please send your inquiry to us through e-mail:sales@yuansu-reagent.com
Usage: Di (2-chloroethyl) amine hydrochloride is an organic compound. It has a wide range of applications in laboratories and industry.
One of the main uses of di (2-chloroethyl) amine hydrochloride is as a coupling reagent in organic synthesis. It can react with carboxylic acids to form amides, and this reaction process is called EDC coupling reaction.
Di (2-chloroethyl) amine hydrochloride can also be used as an intermediate for metal ligands, fluorescent dyes, and lubricants. In organic synthesis, it is
commonly used to catalyze amidation reactions, carboxylation reactions, and deprotection reactions.
Main usages:
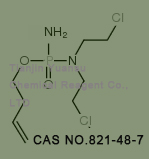
Used for the synthesis of N-[amino(but-3-enoxy)phosphoryl]-2-chloro-N-(2-chloroethyl)ethanamine Cas no. 39800-29-8
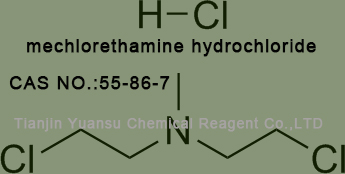
Used for the synthesis of mechlorethamine hydrochloride Cas no. 55-86-7
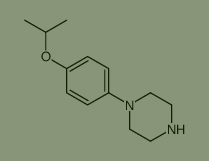
Used for the synthesis of 1-(4-propan-2-yloxyphenyl)piperazine Cas no. 144881-51-6
Besides Safety Information ofBis(2-chloroethyl)amine hydrochloride CAS NO.821-48-7 is also important when handling it
|
Hazard Codes |
Xi |
|
WGK Germany |
3 |
|
H.S.Code: |
2921 1990.90 |
|
TSCA |
Yes |
|
HazardClass |
IRRITANT |
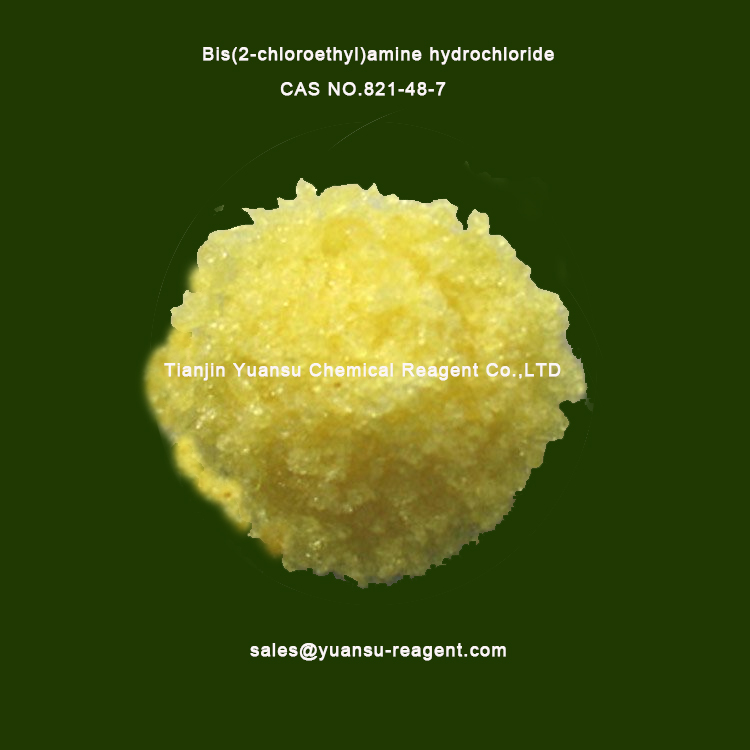
What is the appearance ofBis(2-chloroethyl)amine hydrochloride CAS NO.821-48-7? Please see the picture of Bis(2-chloroethyl)amine hydrochloride CAS NO.821-48-7, below
If you need the products .Please send your inquiry to us through e-mail: sales@yuansu-reagent.com
Specification ofBis(2-chloroethyl)amine hydrochloride CAS NO.821-48-7, is below
Apperance: Colorless to pale yellow crystals with a pungent odor
Assay: 99 min by HPLC
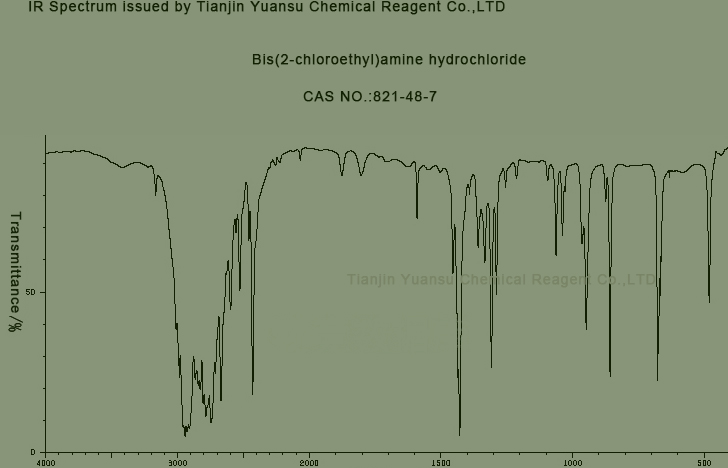
IR identity: conform
IR picture of Bis(2-chloroethyl)amine hydrochloride CAS NO.821-48-7 is as follows,
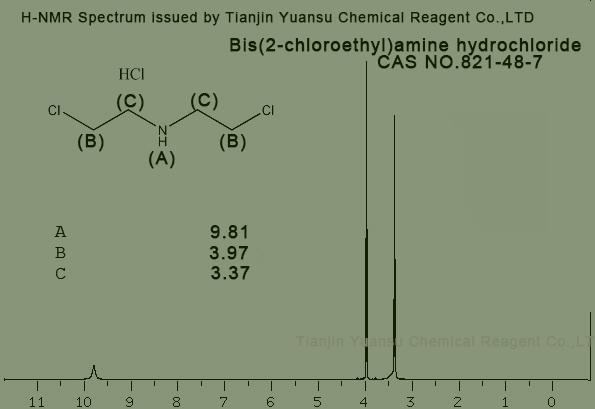
H-NMR Spectrum picture of Bis(2-chloroethyl)amine hydrochloride CAS NO.821-48-7 is as follows,
Reference of Article cited for your reference below,
(1)
Carcinogenicity of chlorinated nitrosotrialkylureas in rats
Publication Date: 1979
Publication Name: Journal of Cancer Research and Clinical Oncology
(2)
Challenges and Opportunities in New Drug Development
Publication Date: 1996
Publication Name: Novel Chemotherapeutic Agents: Preactivation in the Treatment of Cancer and AIDS
(3)
Developmental Approach to Prepare New Types of Antitumor Platinum Complexes with Dual Function
Publication Date: 1991
Publication Name: Platinum and Other Metal Coordination Compounds in Cancer Chemotherapy