4’-Hydroxyacetophenone
What is 4’-Hydroxyacetophenone, cas no:99-93-4,a producer telling you the result.
CAS NO.99-93-4
If you need the products .Please send your inquiry to us through e-mail: sales@yuansu-reagent.com
To begin with, let us tell you what is the basic information of 4’-Hydroxyacetophenone ?
|
Molecular Formula |
C8H8O2 |
Molecular Weight |
136.148 |
|
Density |
1.1±0.1 g/cm3 |
Boiling Point |
313.0±0.0 °C at 760 mmHg |
|
Flash Point |
121.2±12.4 °C |
Melting Point |
132-135 °C(lit.) |

Like many stuff, it has many synonyms as follows
|
EINECS 202-802-8 |
|
MFCD00002355 |
|
QR DV1 |
|
1-(4-Hydroxyphenyl)ethan-1-one |
|
4-Acetylphenol |
|
p-Acetylphenol |
|
Phenol, p-acetyl- |
|
Acetophenone, p-hydroxy- |
|
(4-Hydroxyphenyl)ethan-1-one |
|
1-(4-Hydroxyphenyl)ethanone |
First, the chemical is very special, some technical indexes as below
If you need the products .Please send your inquiry to us through e-mail: sales@yuansu-reagent.com
Physical property data
1. Appearance: White powder
2. Density (g/mL, 25 ℃): 1.109
3. Relative vapor density (g/mL, air=1): not determined
4. Melting point (º C): 109~111
5. Boiling point (º C, atmospheric pressure): 147-148 (399.9pa)
6. Boiling point (º C, 45mmHg): undetermined
7. Refractive index (n109D): 1.5577
8. Flash point (º C): 166
9. Specific rotation (º): not determined
10. Spontaneous combustion point or ignition temperature (º C): not determined
11. Vapor pressure (mmHg, º C): not determined
12. Saturated vapor pressure (kPa, º C): not determined
13. Combustion heat (KJ/mol): undetermined
14. Critical temperature (º C): not determined
15. Critical pressure (KPa): undetermined
16. Logarithmic value of oil-water (octanol/water) partition coefficient: undetermined
17. Explosion upper limit (%, V/V): undetermined
18. Lower explosive limit (%, V/V): not determined
19. Solubility: Slightly soluble in water, easily soluble in ethanol and ether.
toxicology data
1. Acute toxicity: Oral LD50 in mice: 1500mg/kg;
Mouse peritoneal cavity LD50: 200mg/kg;
2. Reproductive toxicity: Oral TDLo: 1mg/kg SEX/DURATION in 11 day pregnant rats;
Ecological data
This substance poses a slight hazard to water.
Molecular structure data
1. Molar refractive index: 38.16
2. Molar volume (cm3/mol): 119.3
3. Waiting ratio (90.2K): 307.4
4. Surface tension (dyne/cm): 43.9
5. Dielectric constant:
6. Polarization distance (10-24cm3):
7. Polarization rate: 15.12
Calculate chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: 7
6. Topological molecule polarity surface area 37.3
7. Number of heavy atoms: 10
8. Surface charge: 0
9. Complexity: 123
10. Number of isotopic atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Nature and stability
1. Avoid contact with oxidants and water.
2. Exists in tobacco leaves and smoke.
Storage method
Store in a cool and ventilated warehouse. Stay away from sources of fire, heat, and water. It should be stored separately from oxidants and avoid mixing storage. Equip with corresponding types and quantities of fire-fighting equipment. The storage area should be equipped with suitable materials to contain leaked materials.
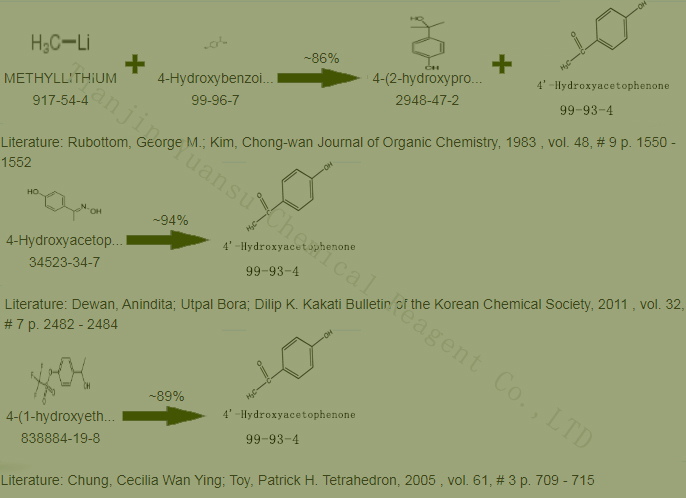
Second, the Synthetic Route we will recommend is the most important for your reference?
First, synthesis line of 4’-Hydroxyacetophenone CAS NO.99-93-4 as follows
synthetic method
Obtained by acylation and transposition of phenol. Mix phenol and acetyl chloride, slowly heat until hydrogen chloride stops escaping, and obtain crude phenyl acetate. Add nitrobenzene, add aluminum trichloride under cooling, and stir at room temperature for 2-3 hours after addition. Then pour it into cold water and add 1:3 hydrochloric acid until it is clear. Extract with ether, recover the ether with the extraction solution, and then perform steam distillation. Nitrobenzene and its byproduct, ortho hydroxyacetophenone, are evaporated by steam at any time, while para hydroxyacetophenone remains in the residue. Extract the residue with ether, recover the ether, cool and crystallize to obtain the crude product, and recrystallize with water to obtain the finished product. The above acylation operation can be performed using acetic anhydride instead of acetyl chloride. Phenol and acetic anhydride are heated and refluxed for 3 hours to produce phenyl acetate with a yield of about 83%. Under the action of anhydrous aluminum trichloride, solvent can also be used for transposition. In addition, p-aminoacetophenone can also be diazotized and hydrolyzed with sodium nitrite to produce 4-hydroxyacetophenone.
Third, what is the usage of 4’-Hydroxyacetophenone CAS NO.99-93-4 ? pleas see below
If you need the products .Please send your inquiry to us through e-mail:sales@yuansu-reagent.com
Usage:
Used as raw materials for manufacturing choleretic drugs and other organic synthesis
Main usages:
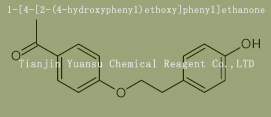
Used for the synthesis of 1-[4-[2-(4-hydroxyphenyl)ethoxy]phenyl]ethanone Cas no. 109720-02-7
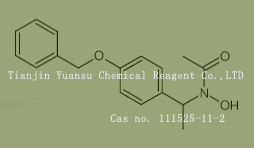
Used for the synthesis of N-hydroxy-N-[1-(4-phenylmethoxyphenyl)ethyl]acetamide Cas no. 111525-11-2
Used for the synthesis of 2-octan-4-yloxy-1-phenylethanone Cas no. 37062-63-8
Besides Safety Information of 4’-Hydroxyacetophenone CAS NO.99-93-4 is also important when handling it
|
Hazard Codes |
Xi |
|
WGK Germany |
3 |
|
H.S.Code: |
2914 5090.90 |
|
TSCA |
Yes |
|
HazardClass |
IRRITANT |
What is the appearance of 4’-Hydroxyacetophenone CAS NO.99-93-4? Please see the picture of 4’-Hydroxyacetophenone CAS NO.99-93-4, below
If you need the products .Please send your inquiry to us through e-mail: sales@yuansu-reagent.com
Specification of 4’-Hydroxyacetophenone CAS NO.99-93-4, is below
Apperance: white crystalline powder
Assay: 99 min by HPLC
IR identity: conform
IR picture of 4’-Hydroxyacetophenone CAS NO.99-93-4 is as follows,
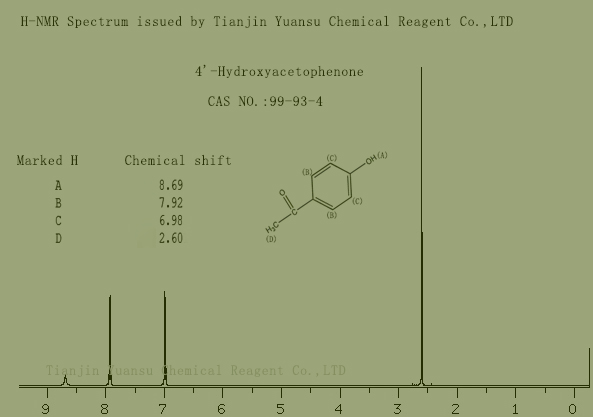
H-NMR Spectrum picture of 4’-Hydroxyacetophenone CAS NO.99-93-4 is as follows,
Reference of Article cited for your reference below,
(1)
Compounds derived from aminoacetic acids
Publication Date: 2005
Publication Name: Handbook of Hydroxyacetophenones: Preparation and Physical Properties
(2)
Publication Date: 2022
Publication Name: Synlett
(3)
DOI: 10.1002/aoc.3328
Publication Date: 2015
Publication Name: Appl. Organometal. Chem.