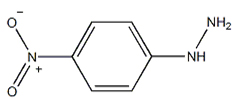
4-Nitrophenylhydrazine
What is 4-Nitrophenylhydrazine, cas no:100-16-3,a producer telling you the result.
CAS NO.100-16-3
If you need the products .Please send your inquiry to us through e-mail: sales@yuansu-reagent.com
To begin with, let us tell you what is the basic information of 4-Nitrophenylhydrazine ?
|
Molecular Formula |
C6H7N3O2 |
Molecular Weight |
153.139 |
|
Density |
1.4±0.1 g/cm3 |
Boiling Point |
344.0±25.0 °C at 760 mmHg |
|
Flash Point |
161.8±23.2 °C |
Melting Point |
156 °C (dec.)(lit.) |
Like many stuff, it has many synonyms as follows
|
Hydrazine, (4-nitrophenyl)- |
|
4-Nitrophenylhydrazine |
|
4-Nitropheylhydrazine |
|
(p-Nitrophenyl)hydrazine |
First, the chemical is very special, some technical indexes as below
If you need the products .Please send your inquiry to us through e-mail: sales@yuansu-reagent.com
Physical property data
1. Appearance: Orange yellow crystalline powder [1]
2. Melting point (℃): 156 (decomposition) [2]
3. Octanol/water partition coefficient: 1.41 [3]
4. Solubility: Slightly soluble in water, soluble in hot water, benzene, ethanol, ether, chloroform, and ethyl acetate. [4]
toxicology data
1. Acute toxicity: LDLo in mouse peritoneal cavity: 250mg/kg;
2. Mutability: Microbial mutation test: bacteria Salmonella typhimurium, 50 μ g/plate; Mutation Microbial Test: Microbial, 780 μ g/L.
3. There is currently no data available on acute toxicity
4. There is currently no data available on irritability
5. Other [5] LDLo: 250mg/kg (mouse abdominal cavity)
Ecological data
1. There is currently no information on ecological toxicity
2. There is currently no data available on biodegradability
3. There is currently no information available on non biodegradable materials
Molecular structure data
1. Molar refractive index: 41.31
2. Molar volume (cm3/mol): 107.9
3. Waiting for Zhang Biarong (90.2K): 310.8
4. Surface tension (dyne/cm): 68.9
5. Dielectric constant:
6. Polarization distance (10-24cm3):
7. Polarization rate: 16.37
Calculate chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 2
3. Number of hydrogen bond acceptors: 4
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: None
6. Topological molecule polarity surface area 83.9
7. Number of heavy atoms: 11
8. Surface charge: 0
9. Complexity: 137
10. Number of isotopic atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Nature and stability
1. Stability [6] Stability
2. Prohibited compounds [7] Strong oxidizing agents, strong reducing agents, strong acids
3. Conditions to avoid contact [8] Friction, vibration, or impact
4. Aggregation hazard [9] Non aggregation
5. Decomposition products [10] Nitrogen oxides
Storage method
Storage precautions [11] Store in a cool and ventilated warehouse. Stay away from sources of fire and heat. Sealed packaging. It should be stored separately from oxidants, reducing agents, acids, and edible chemicals, and avoid mixing storage. Equip with corresponding types and quantities of fire-fighting equipment. The storage area should be equipped with suitable materials to contain leaked materials.
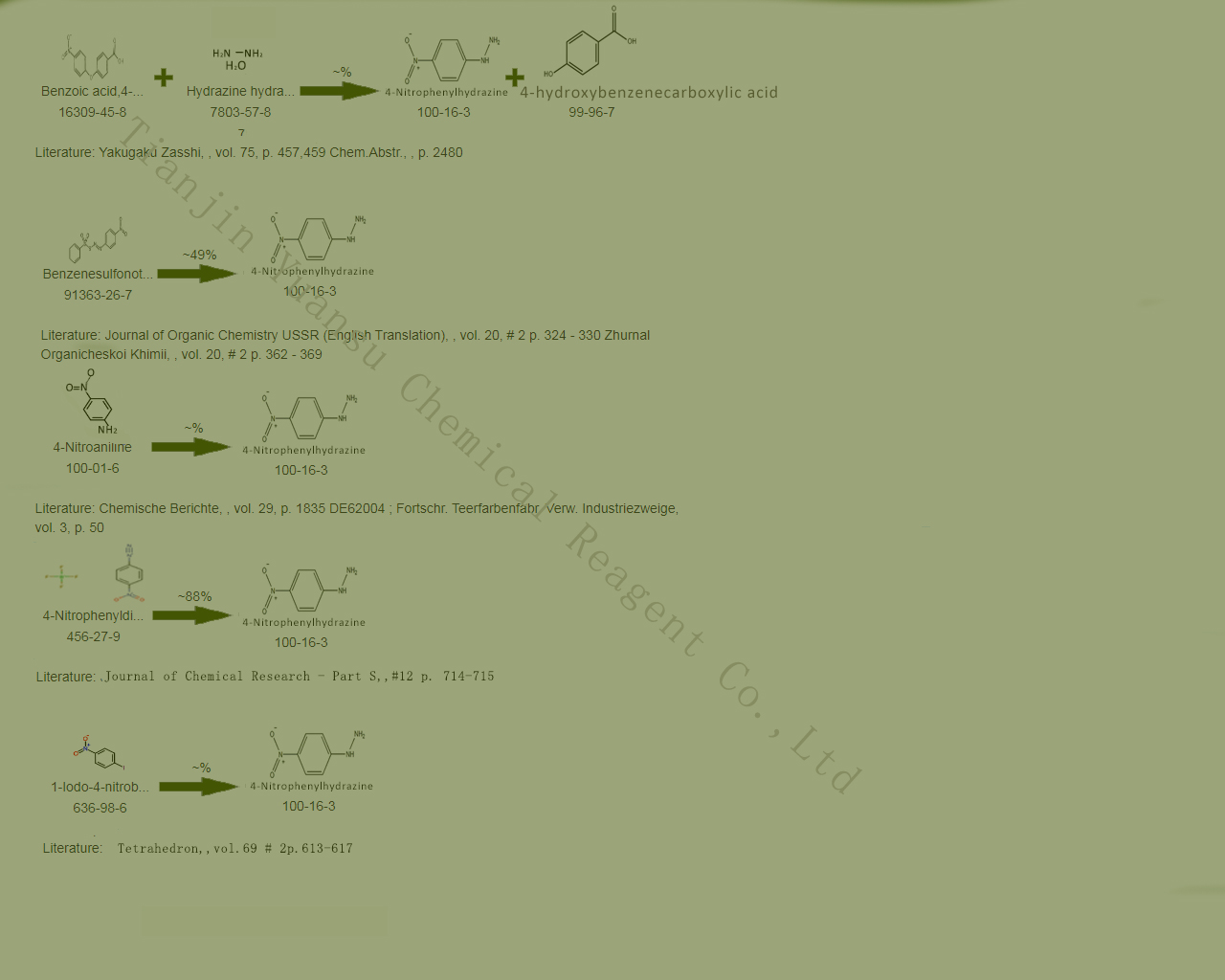
Second, the Synthetic Route we will recommend is the most important for your reference?
First, synthesis line of 4-Nitrophenylhydrazine CAS NO.100-16-3 as follows
Main production methods:
Dissolve p-nitroaniline in hydrochloric acid, cool to 0 ℃, add sodium nitrite solution for diazotization, and filter. Add the filtrate to a cooled mixture of sodium sulfite and sodium hydroxide, then add concentrated hydrochloric acid to make it acidic. Warm it at 25 ℃ for half an hour and filter to obtain p-nitrophenylhydrazine hydrochloride. Dissolve in water and add sodium acetate deep solution to precipitate p-nitrophenylhydrazine with a yield of 63-72%.
Third, what is the usage of 4-Nitrophenylhydrazine CAS NO.100-16-3 ? pleas see below
If you need the products .Please send your inquiry to us through e-mail:sales@yuansu-reagent.com
Main usages: Used as a reagent for testing ketones, aldehydes, and sugars.
Other usages:
Used for the synthesis of 3-(4-nitrophenyl)pyridine Cas no. 4282-46-6
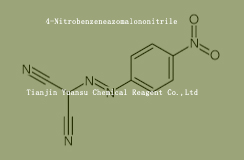
Used for the synthesis of 4-Nitrobenzeneazomalononitrile Cas no. 1080-02-0
Used for the synthesis of nitrobenzene Cas no. 98-95-3
Besides Safety Information of 4-Nitrophenylhydrazine CAS NO.100-16-3 is also important when handling it
|
Hazard Codes |
Xi |
|
WGK Germany |
3 |
|
H.S.Code: |
2928 0000.90 |
|
TSCA |
Yes |
|
HazardClass |
IRRITANT |
What is the appearance of 4-Nitrophenylhydrazine CAS NO.100-16-3? Please see the picture of 4-Nitrophenylhydrazine CAS NO.100-16-3, below
If you need the products .Please send your inquiry to us through e-mail: sales@yuansu-reagent.com
Specification of 4-Nitrophenylhydrazine CAS NO.100-16-3, is below
Apperance: Orange yellow crystalline powder
Assay: 99% min by HPLC
IR identity: conform
Melting point: 156 to 157 °C
IR picture of 4-Nitrophenylhydrazine CAS NO.100-16-3 is as follows,
If you need the IR Spectrum please send your e-mail to : sales@yuansu-reagent.com
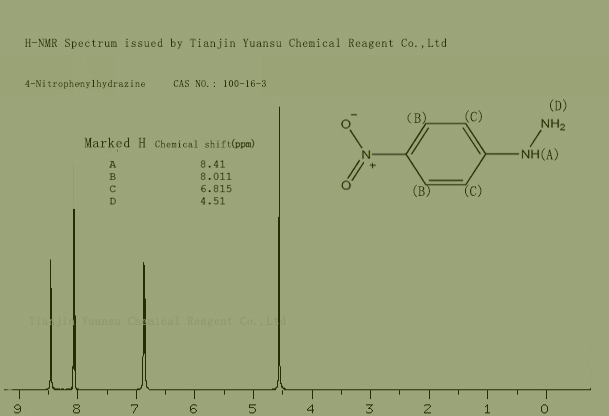
HNMR Spectrum picture of 4-Nitrophenylhydrazine CAS NO.100-16-3 is as follows,
Reference of Article cited for your reference below,
(1)
Durchflussunterstützte Synthese von Heterocyclen bei hohen Temperaturen
Publication Name: Flow-Chemie für die Synthese von Heterocyclen
Publication Date: 2024
DOI: 10.1007/978-3-031-51912-3_4
(2)
Publication Name: Chemical Papers
Publication Date: 2022-12-09
DOI: 10.1007/s11696-022-02618-x
(3)
Publication Date: 2007
Publication Name: Journal of Thermal Analysis and Calorimetry