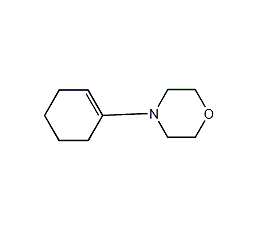
N-(1-Cyclohexen-1-yl)morpholine
What is N-(1-Cyclohexen-1-yl)morpholine, cas no:670-80-4,a producer telling you the result.
CAS NO.670-80-4
If you need the products .Please send your inquiry to us through e-mail: sales@yuansu-reagent.com
To begin with, let us tell you what is the basic information of N-(1-Cyclohexen-1-yl)morpholine ?
|
Molecular Formula |
C10H17NO |
Molecular Weight |
167.248 |
|
Density |
1.0±0.1 g/cm3 |
Boiling Point |
249.7±0.0 °C at 760 mmHg |
|
Flash Point |
68.3±0.0 °C |
Exact Mass |
167.131012 |
Like many stuff, it has many synonyms as follows
|
EINECS 211-579-6 |
|
MFCD00006163 |
|
Morpholine, 4-(1-cyclohexen-1-yl)- |
|
4-(cyclohexen-1-yl)morpholine |
|
4-(1-Cyclohexen-1-yl)morpholine |
|
Morpholine, 4- (1-cyclohexen-1-yl)- |
|
4-(1-Cyclohexenyl)morpholine |
|
4-(Cyclohex-1-en-1-yl)morpholine |
|
4-(cyclohex-1-enyl)morpholine |
|
N-(1-Cyclohexenyl)morpholine |
| Morpholino-1-cyclohexene |
First, the chemical is very special, some technical indexes as below
If you need the products .Please send your inquiry to us through e-mail: sales@yuansu-reagent.com
Physical property data
1. Appearance: Colorless liquid.
2. Density (g/mL, 25/4 ℃): 0.995
3. Relative vapor density (g/mL, air=1): not determined
4. Melting point (º C): undetermined
5. Boiling point (º C, atmospheric pressure): undetermined
6. Boiling point (º C, 5.2kPa): undetermined
7. Refractive index: 1.514
8. Flash point (º C): 68
9. Specific rotation (º): not determined
10. Spontaneous combustion point or ignition temperature (º C): not determined
11. Vapor pressure (kPa, 25 º C): not determined
12. Saturated vapor pressure (kPa, 60 º C): not determined
13. Combustion heat (KJ/mol): undetermined
14. Critical temperature (º C): not determined
15. Critical pressure (KPa): undetermined
16. Logarithmic value of oil-water (octanol/water) partition coefficient: undetermined
17. Explosion upper limit (%, V/V): undetermined
18. Lower explosive limit (%, V/V): not determined
19. Solubility: Insoluble in water.
toxicology data
Currently unavailable
Ecological data
Do not allow undiluted or large quantities of products that are slightly harmful to water to come into contact with groundwater, waterways, or sewage systems. Do not discharge materials into the surrounding environment without government permission.
Molecular structure data
1. Molar refractive index: 48.93
2. Molar volume (m3/mol): 159.9
3. Waiting for Zhang Biarong (90.2K): 402.0
4. Surface tension (dyne/cm): 39.9
5. Dielectric constant:
6. Polarization distance (10-24cm3):
7. Polarization rate: 19.39
Calculate chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 1.7
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: None
6. Topological molecular polarity surface area 12.5
7. Number of heavy atoms: 12
8. Surface charge: 0
9. Complexity: 171
10. Number of isotopic atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Nature and stability
Stay away from oxides and strong acids.
Storage method
Store in a sealed container and keep in a cool, dry place. The storage location must be kept away from oxidants.
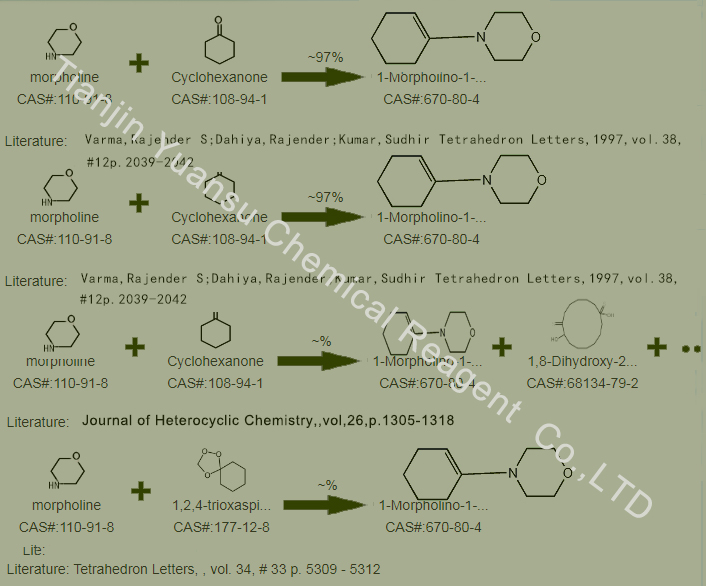
Second, the Synthetic Route we will recommend is the most important for your reference?
There are multiple ways to prepare 1-methylmorpholine-1-cyclohexene, one commonly used method is to react cyclohexene with morpholine under alkaline conditions. Add cyclohexene and morpholine to the reactor and react under alkaline conditions for a period of time. Then, through appropriate purification steps, pure 1-morpholino-1-cyclohexene product can be obtained.
First, synthesis line of N-(1-Cyclohexen-1-yl)morpholine CAS NO.670-80-4 as follows
Third, what is the usage of N-(1-Cyclohexen-1-yl)morpholine CAS NO.670-80-4 ? pleas see below
If you need the products .Please send your inquiry to us through e-mail:sales@yuansu-reagent.com
Usage:
1-morpholino-1-cyclohexene has various applications in the field of chemistry. It can be used as a solvent, as a catalyst and reducing agent in organic synthesis. It can also serve as a chemical reagent and intermediate, participating in various organic synthesis reactions.
Other usage:
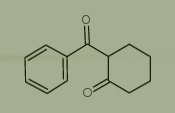
Used for the synthesis of 2-Benzoylcyclohexanone, Cas no. 3580-38-9
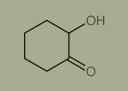
Used for the synthesis of 2-Hydroxycyclohexanone, Cas no. 533-60-8
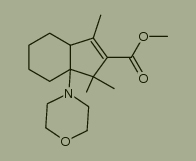
Used for the synthesis of methyl 1,1,3-trimethyl-7a-morpholino-3a,4,5,6,7,7a-hexahydro-1H-indene-2-carboxylate Cas no. 108790-85-8
Besides Safety Information of N-(1-Cyclohexen-1-yl)morpholine CAS NO.670-80-4 is also important when handling it
|
Hazard Codes |
Xi |
|
WGK Germany |
3 |
|
H.S.Code: |
2934999090 |
|
TSCA |
Yes |
|
HazardClass |
IRRITANT |
What is the appearance of N-(1-Cyclohexen-1-yl)morpholine CAS NO.670-80-4? Please see the picture of N-(1-Cyclohexen-1-yl)morpholine CAS NO.670-80-4, below
If you need the products .Please send your inquiry to us through e-mail: sales@yuansu-reagent.com
Specification of N-(1-Cyclohexen-1-yl)morpholine CAS NO.670-80-4, is below
Apperance: Colorless to Light yellow to Light orange clear liquid.
Assay: 97 min by GC
IR identity: conform
Water:0.5% max by K.F.
IR picture of N-(1-Cyclohexen-1-yl)morpholine CAS NO.670-80-4 is as follows,
Reference of Article cited for your reference below,
(1)
Publication Name: Understanding the Microbiome Interactions in Agriculture and the Environment
Publication Date: 2022
DOI: 10.1007/978-981-19-3696-8_16
(2)
Publication Name: Synlett
Publication Date: 2020-04-06
(3)
Hydrogenation of Olefins, Alkynes, Allenes, and Arenes by Borane-Based Frustrated Lewis Pairs
Publication Name: Synthesis
Publication Date: 2021-12-22
DOI: 10.1055/a-1684-5552