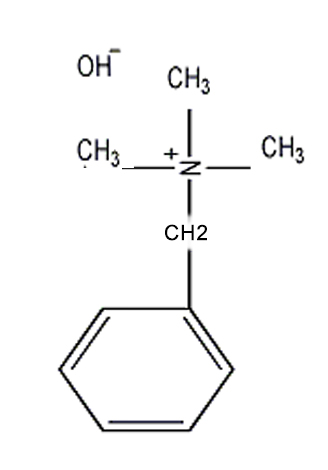
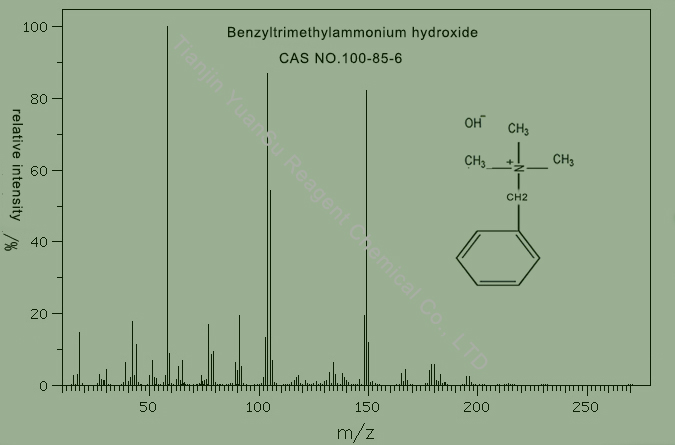
Benzyltrimethylammonium hydroxide,
What is Benzyltrimethylammonium hydroxide, cas no:100-85-6,a producer telling you the result.
CAS NO.100-85-6
If you need the products .Please send your inquiry to us through e-mail: sales@yuansu-reagent.com
To begin with, let us tell you what is the basic information of Benzyltrimethylammonium hydroxide ?
|
Flash Point |
60 °F |
Molecular Weight |
167.25 |
|
Density |
1.059 g/mL at 25 °C |
Boiling Point |
65°C |
|
Molecular Formula |
C10H17NO |
Melting Point |
-98ºC |
Like many stuff, it has many synonyms as follows
|
MFCD00008281 |
|
Benzyltrimethylammoniumhydrox |
|
N,N,N-trimethylbenzenemethanaminium hydroxide |
|
Benzyltrimethylammoaium bydroxide |
|
TRITON B |
|
benzyltrimethyl-ammoniuhydroxide |
|
benzyl-trimethylammonium hydroxide |
|
N-benzyl-trimethylammonium hydroxide |
|
sumquat2311 |
|
Benzyltrimethylammonium hydroxide |
|
Trimethylbenzylammonium hydroxide |
|
Benzyl trimethyl ammonium hydroxide |
|
trimethyl-benzyl-ammonium hydroxide |
| Benzyl-trimethylazanium hydroxide |
First, the chemical is very special, some technical indexes as below
If you need the products .Please send your inquiry to us through e-mail: sales@yuansu-reagent.com
Physical property data
1. Appearance: Colorless to pale yellow transparent liquid
2. Density (g/mL, 25 ℃): 1.059
3. Relative vapor density (g/mL, air=1): not determined
4. Melting point (º C): undetermined
5. Boiling point (º C, atmospheric pressure): 65
6. Boiling point (º C, 61KPa): undetermined
7. Refractive index: 1.43
8. Flash point (º C): 15
9. Specific rotation (º): not determined
10. Spontaneous combustion point or ignition temperature (º C): not determined
11. Vapor pressure (mmHg, 20 º C): not determined
12. Saturated vapor pressure (kPa, 20 º C): not determined
13. Combustion heat (KJ/mol): undetermined
14. Critical temperature (º C): not determined
15. Critical pressure (KPa): undetermined
16. Logarithmic value of oil-water (octanol/water) partition coefficient: undetermined
17. Explosion upper limit (%, V/V): undetermined
18. Lower explosive limit (%, V/V): not determined
19. Solubility: soluble in methanol and water.
toxicology data
Acute toxicity: Subcutaneous LDL0:35mg/kg in mice;
Ecological data
This substance is harmful to the environment and special attention should be paid to its pollution of water bodies.
Molecular structure data
Currently unavailable
Calculate chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 2
5. Number of tautomers: None
6. Topological molecular polarity surface area 1
7. Number of heavy atoms: 12
8. Surface charge: 0
9. Complexity: 107
10. Number of isotopic atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 2
Nature and stability
Avoid contact with oxides and acids.
Storage method
Store in a cool and ventilated warehouse. Stay away from sources of fire, heat, and static electricity. The storage temperature should not exceed 30 ℃. It should be stored separately from oxidants and acidic substances, and avoid mixing storage. Sealed storage. Not suitable for storing in large quantities
To store or preserve for a long time. Adopt explosion-proof lighting and ventilation facilities. Prohibit the use of mechanical equipment and tools that are prone to generating sparks. The storage area should be equipped with emergency response equipment for leaks and suitable containment materials.
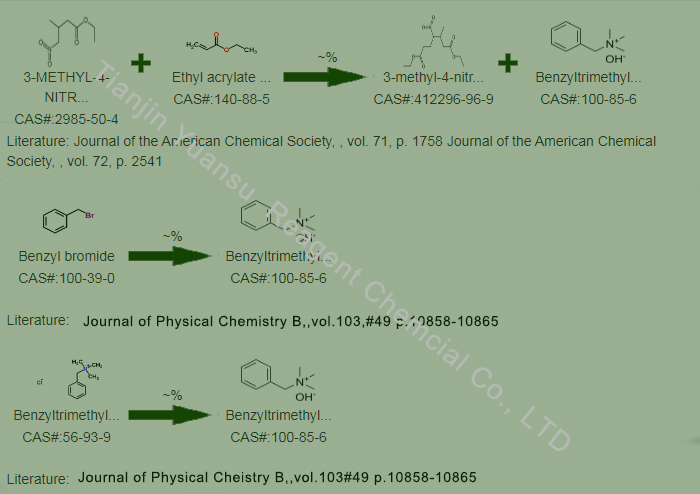
Second, the Synthetic Route we will recommend is the most important for your reference?
First, synthesis line of Benzyltrimethylammonium hydroxide CAS NO.100-85-6 as follows


Manufacturing method:
Benzyltrimethylammonium hydroxide can be prepared by reacting trimethylamine with benzyl bromide. The specific synthesis method includes reacting trimethylamine with benzyl bromide in an alkaline solution to produce benzyl trimethylamine, which is then reacted with sodium hydroxide or calcium hydroxide to obtain the final product.
Third, what is the usage of Benzyltrimethylammonium hydroxide CAS NO.100-85-6 ? pleas see below
If you need the products .Please send your inquiry to us through e-mail:sales@yuansu-reagent.com
Ion exchange agent: Benzyltrimethylammonium hydroxide can be used as an ion exchange agent for treating organic ions, metal ions, and inorganic ions in water. It can remove some harmful substances such as heavy metal ions from water.
Surfactant: Due to its hydrophilic and hydrophobic properties, benzyltrimethylammonium hydroxide can be used as a surfactant and is widely used in the synthesis of detergents, antibacterial agents, fluorescent whitening agents, and other fields.
Preservative: Benzyltrimethylammonium hydroxide can also be used as a preservative and added to some personal care products to extend their shelf life.
Other usage:
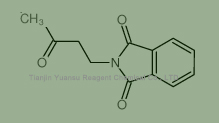
Used for the synthesis of 2-(3-oxobutyl)isoindole-1,3-dione, Cas no. 3783-77-5
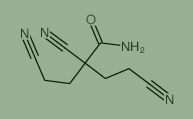
Used for the synthesis of 2,4-dicyano-2-(2-cyanoethyl)butanamide, Cas no. 1112-50-1
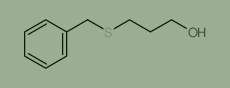
Used for the synthesis of 3-benzylsulfanylpropan-1-ol Cas no. 26902-03-4
Besides Safety Information of Benzyltrimethylammonium hydroxide CAS NO.100-85-6 is also important when handling it
|
Hazard Codes |
Xi |
|
WGK Germany |
3 |
|
H.S.Code: |
2923 9000.90 |
|
TSCA |
Yes |
|
HazardClass |
IRRITANT |
What is the appearance of Benzyltrimethylammonium hydroxide CAS NO.100-85-6? Please see the picture of Benzyltrimethylammonium hydroxide CAS NO.100-85-6, below
If you need the products .Please send your inquiry to us through e-mail: sales@yuansu-reagent.com
Specification of Benzyltrimethylammonium hydroxide CAS NO.100-85-6, is below
Apperance: colorless to light yellow liquid.
Assay: 99 min by GC
IR identity: conform
Water:0.5% max by K.F.
mass spectrum of Benzyltrimethylammonium hydroxide CAS NO.100-85-6 is as follows,
Reference of Article cited for your reference below,
(1)
Publication Name: Physical chemistry chemical physics : PCCP
Publication Date: 2023-07-26
PMID: 37462948
DOI: 10.1039/d3cp01380d
(2)
Publication Name: Chemical Papers
Publication Date: 2022-04-26
DOI: 10.1007/s11696-022-02217-w
(3)
Publication Name: npj Computational Materials
Publication Date: 2023-11-09