4-(3-Chloro-4-fluorophenylamino)-7-methoxyquinazolin-6-ol
What is 4-(3-Chloro-4-fluorophenylamino)-7-methoxyquinazolin-6-ol, cas no. 184475-71-6,a producer telling you the result.
CAS NO. 184475-71-6
If you need the products .Please send your inquiry to us through e-mail: sales@yuansu-reagent.com
To begin with, let us tell you what is the basic information of 4-(3-Chloro-4-fluorophenylamino)-7-methoxyquinazolin-6-ol CAS NO. 184475-71-6 ?
|
Molecular Formula |
C15H11ClFN3O2 |
Molecular Weight |
319.718 |
|
Density |
1.5±0.1 g/cm3 |
Boiling Point |
478.8±45.0 °C at 760 mmHg |
|
Flash Point |
243.4±28.7 °C |
Melting Point |
>260ºC (dec.) |
Like many stuff, it has many synonyms as follows
|
FAAH-IN-2 |
|
Gefitinib Impurity 2 |
|
4-[(3-chloro-4-fluorophenyl)amino]-7-methoxyquinazolin-6-ol |
|
4-(3-chloro-4-fluoroanilino)-7-methoxyquinazolin-6-ol |
|
4-(3-chloro-4-fluorophenylamino)-7-methoxy quinazolin-6-ol |
|
6-Quinazolinol, 4-[(3-chloro-4-fluorophenyl)amino]-7-methoxy- |
First, the chemical is very special, some technical indexes as below
If you need the products .Please send your inquiry to us through e-mail: sales@yuansu-reagent.com
Second, the Synthetic Route we will recommend is the most important for your reference?
First, synthesis line of 4-(3-Chloro-4-fluorophenylamino)-7-methoxyquinazolin-6-ol CAS NO. 184475-71-6 as follows
Manufacture method:
-The preparation method of 4- (3-chloro-4-fluoroaniline) -7-methoxy-quinazolin-6-ol usually includes the following steps:
1. Using appropriate reaction conditions, react 3-chloro-4-fluoroaniline with 7-methoxy-quinazolin-6-ol to generate the target compound.
2. Perform crystallization and purification steps to obtain single crystals of the target compound.
Third, what is the usage of 4-(3-Chloro-4-fluorophenylamino)-7-methoxyquinazolin-6-ol CAS NO. 184475-71-6 ? pleas see below
If you need the products .Please send your inquiry to us through e-mail:sales@yuansu-reagent.com
Usage:
184475-71-6 is a chemical substance with a purity of 99%, mainly used as an intermediate in the preparation of gefitinib. Gefitinib is a medication used to treat certain types of cancer. This compound, 4- (3-chloro-4-fluorophenylamino) -7-methoxyquinazolin-6-ol, is a key component in the synthesis of gefitinib. Its molecular formula is C15H11ClFN3O2, and its appearance is a light brown to off white powder. In addition, this compound is also used to supply gefitinib API (active pharmaceutical ingredient) and intermediates, demonstrating its important position in drug development and production.
Other usages as below
Used for the synthesis of Gefitinib Cas no. 184475-35-2
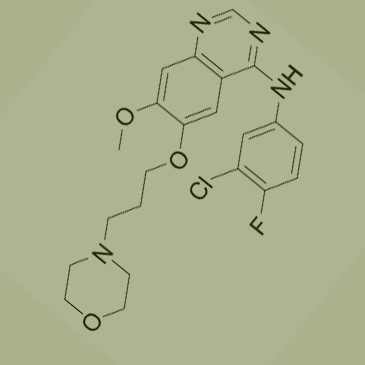
Used for the synthesis of N-(3-Chloro-4-fluorophenyl)-6-(3-chloropropoxy)-7-methoxyquinazolin-4-amine Cas no. 912556-91-3
Used for the synthesis of 6-Hydroxy-7-methoxy-3,4-dihydroquinazolin-4-one Cas no. 179688-52-9
Besides Safety Information of 4-(3-Chloro-4-fluorophenylamino)-7-methoxyquinazolin-6-ol CAS NO.184475-71-6 is also important when handling it
|
Hazard Codes |
Xi |
|
WGK Germany |
3 |
|
H.S.Code: |
2933 9900.99 |
|
TSCA |
Yes |
|
HazardClass |
IRRITANT |
What is the appearance of 4-(3-Chloro-4-fluorophenylamino)-7-methoxyquinazolin-6-ol CAS NO.184475-71-6? Please see the picture of 4-(3-Chloro-4-fluorophenylamino)-7-methoxyquinazolin-6-ol CAS NO.184475-71-6, below
If you need the products .Please send your inquiry to us through e-mail: sales@yuansu-reagent.com
Specification of 4-(3-Chloro-4-fluorophenylamino)-7-methoxyquinazolin-6-ol CAS NO.184475-71-6, is below
Appearance: white or white like crystalline substance
Solubility: It is soluble in most organic solvents such as ethanol,
dimethylformamide, and chlorinated hydrocarbons at room temperature.
Assay:99% min by GC.
IR Identity: conform.
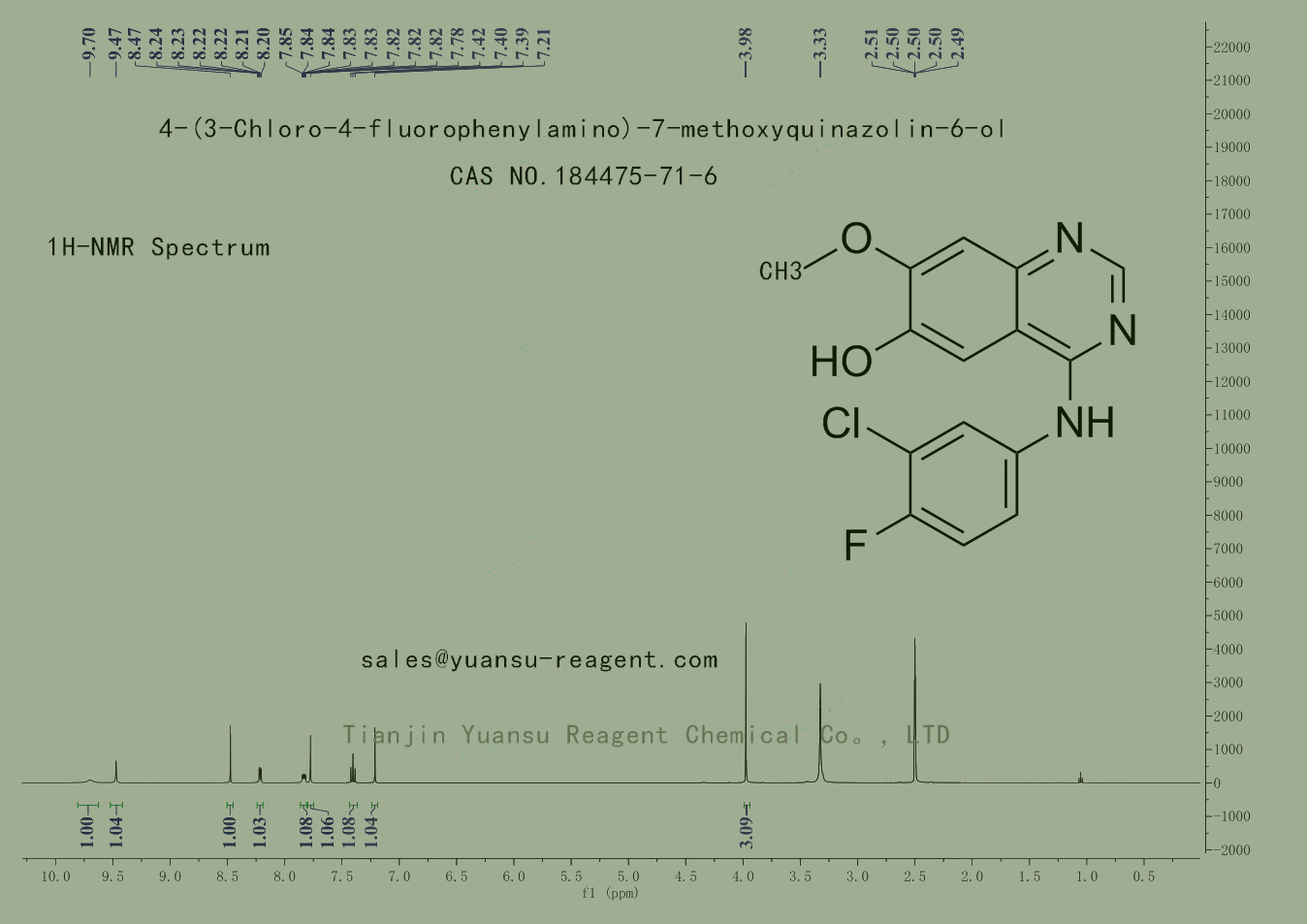
HNMR picture of 4-(3-Chloro-4-fluorophenylamino)-7-methoxyquinazolin-6-ol CAS NO.184475-71-6 is as follows,
Reference of Article cited for your reference below,
(1)
Egfr protein degradant and anti-tumor application thereof
Publication Number: US-2022313829-A1
Priority Date: 2019-08-05
(2)
A kind of isoindolinone-containing quinazoline-based carboxylate derivatives and application thereof
Publication Number: CN-112125890-B
Priority Date: 2020-09-25
Grant Date: 2022-12-06