3,4-Dihydro-2H-pyran
What is 3,4-Dihydro-2H-pyran, cas no. 110-87-2,a producer telling you the result.
CAS NO. 110-87-2
If you need the products .Please send your inquiry to us through e-mail: sales@yuansu-reagent.com
To begin with, let us tell you what is the basic information of 3,4-Dihydro-2H-pyran CAS NO. 110-87-2 ?
|
Molecular Formula |
C5H8O |
Molecular Weight |
84.116 |
|
Density |
0.9±0.1 g/cm3 |
Boiling Point |
86.5±0.0 °C at 760 mmHg |
|
Flash Point |
-15.6±0.0 °C |
Melting Point |
−70 °C(lit.) |
Like many stuff, it has many synonyms as follows
|
EINECS 203-810-4 |
|
MFCD00006558 |
|
3,4-2H-dihydropyran |
|
5,6-DIHYDRO-4H-PYRAN |
|
Dihydro-2H-pyran |
|
2,3-dihydropyran |
|
pyran,dihydro |
|
DHP |
|
2,3-Dihydro-4H-p yran |
|
DIHYDROPYRANE |
|
2H-Pyran, 3,4-dihydro- |
|
3,4-Dihydro-2H-pyran (DHP) |
|
2H-3,4-dihydropyran |
|
Pyran, 2,3-dihydro- |
|
3,4-Dihydro-2H-pyran |
|
3,4-dihydropyran |
|
Dihydropyran |
First, the chemical is very special, some technical indexes as below
If you need the products .Please send your inquiry to us through e-mail: sales@yuansu-reagent.com
1. Appearance: Colorless and easy to flow liquid
2. Relative density (g/mL, 20/4 ℃): 0.922
3. Relative vapor density (g/mL, air=1): 2.9
4. Melting point (º C): -70
5. Boiling point (º C, atmospheric pressure): 86.5
6. Refractive index (n20 º C): 1.4423
7. Flash point (º C, closed): -15
8. Self ignition point or ignition temperature (º C): 240
9. Saturated vapor pressure (kPa, 20 º C): 120
10. Explosion upper limit (%, V/V): 13.8
11. Lower explosive limit (%, V/V): 1.1
12. Solubility: Can be mixed with ethanol, ether, and general organic solvents, and slightly soluble in water.
13. Room temperature refractive index (n25): 1.4400
14. Critical temperature (º C): 288.85
15. Critical pressure (MPa): 4.56
16. Critical density (g · cm-3): 0.314
17. Critical volume (cm3 · mol-1): 268
18. Critical compression factor: 0.262
19. Eccentricity factor: 0.247
20. Freezing point (º C): -15
21. Solubility: soluble in water and ethanol, slightly soluble in chloroform.
toxicology data
1. Acute toxicity:
Rat oral LD50:>4000mg/kg;
Inhalation of LC50 in rats:>10700mg/m3/4H
2. Irritation: Rabbit transdermal, moderate irritation. Rabbit eyes, moderate irritation.
Ecological data
This substance may be harmful to the environment, and special attention should be paid to its pollution of water bodies.
Molecular structure data
1. Molar refractive index: 17.20
2. Molar volume (cm3/mol): 70.6
3. Waiting for Zhang Biarong (90.2K): 157.2
4. Surface tension (dyne/cm): 24.5
5. Polarization rate (10-24cm3): 6.82
Calculate chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: None
6. Topological molecular polarity surface area 9.2
7. Number of heavy atoms: 6
8. Surface charge: 0
9. Complexity: 57
10. Number of isotopic atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Nature and stability
1. It does not decompose at room temperature and pressure. Do not come into contact with strong oxidants and avoid heating. Flammable, can cause combustion or explosion when exposed to high heat, open flames, and oxidants.
When in contact with peroxides, sensitive peroxides are generated. Purifying these compounds can cause violent explosions, and separating these products requires great caution.
3. Form of existence: Exists in tobacco leaves.
Storage method
Store in a cool and ventilated warehouse. Stay away from sources of fire, heat, and static electricity. The storage temperature should not exceed 30 ℃. It should be stored separately from oxidants and avoid mixing storage. Adopt explosion-proof lighting and ventilation facilities. Prohibit the use of mechanical equipment and tools that are prone to generating sparks. The storage area should be equipped with emergency response equipment for leaks and suitable containment materials.
Second, the Synthetic Route we will recommend is the most important for your reference?
First, synthesis line of 3,4-Dihydro-2H-pyran CAS NO. 110-87-2 as follows
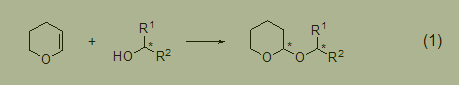
Tetrahydropyranose (THP) of alcohols is a very useful tool for protecting alcohols in organic synthesis. The reaction between chiral alcohols and dihydropyrans introduces another asymmetric center, resulting in a diastereomeric mixture (Formula 1). This poses certain difficulties for purification and spectral analysis, but does not hinder its successful application.
Under very mild reaction conditions (0 oC, 1 h, 89%~100%), the use of (trimethylsilyl) sulfate can cause the tetrahydropyranylation reaction of alcohols, and even with the use of acrylic tertiary alcohols, no rearrangement reaction occurs.
The decomposition of tetrahydropyran derivatives in the presence of MEM ether, MOM ether, and 1,3-dioxolane is highly effective in the selective cleavage of TEM ether by organotin phosphate condensation reaction, and the catalyst can be reused. In the presence of tert butyldimethylsilyl, acetyl, methylsulfonyl, and methoxymethyl ether, as well as the methylsulfonyl catalyst Me2Sn (SME) 2-BF3 · Et2O, THP ethers of primary, secondary, and tertiary alcohols undergo selective cleavage [2].
The tetrahydropyranose derivatives of thiols can be used in functional group shielding reactions (Formula 2).
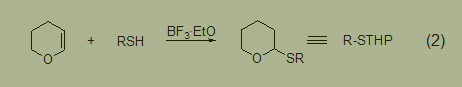
Compared with O-tetrahydropyran ether, S-tetrahydropyran ether can also exist stably under 4mol/L HCl MeOH conditions. The deprotection reaction using silver nitrate or hydrogen bromide trifluoroacetic acid can be easily carried out with high yields. Oxidation reactions can occur using iodine, cyanide sulfide, or disulfide.
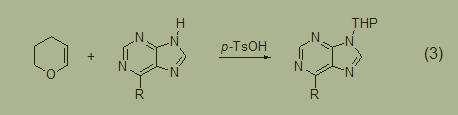
The tetrahydropyranization of amines involves the reaction of purine with dihydropyran in the presence of catalytic amounts of p-toluenesulfonic acid, resulting in the formation of 9- (2-tetrahydropyran) derivatives (Formula 3) [4].
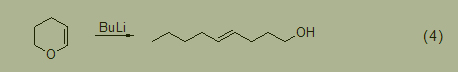
The ring opening reaction of dihydropyran is carried out by treating dihydropyran with sodium pentyl or n-butyl lithium to generate trans 4-hydroxy-1-ol (Formula 4) [5].
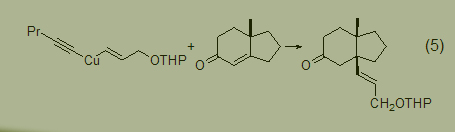
In organic synthesis, groups protected by THP have a wide range of applications. The use of Gilman cuprate reagent with chain end protecting ether can introduce the structure of cis-CH=CHCH2OH (Formula 5) [6].
The pyrimidine compound generated by dihydropyran and BrCH2C (NOH) CO2Et can be applied in the synthesis of amino triesters (Formula 6) [7].
A simple, effective, and highly chemically selective method for tetrahydropyranylation of alcohols and phenolic compounds is carried out at room temperature using polystyrene supported on a catalytic amount of AlCl3. This method has high selectivity for the single protection of symmetrical diols (Formula 7) [8].
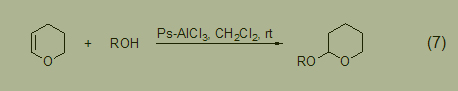
Third, what is the usage of 3,4-Dihydro-2H-pyran CAS NO. 110-87-2 ? pleas see below
If you need the products .Please send your inquiry to us through e-mail:sales@yuansu-reagent.com
Main usage:
1. Tetrahydropyran, pentanediol, glutaric acid, valerolactone, pentadiene, and resin products can be prepared through polymerization, hydrogenation, oxidation, and other reactions. It can also be used as a pharmaceutical intermediate. Solvent, organic synthesis intermediate.
2. Widely used for protecting hydroxyl groups, it generally does not react with nucleophiles and organometallic reagents, is resistant to strong bases, and is prone to change under low pH or Lewis acid conditions.
Other usages as below
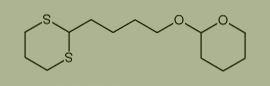
Used for the synthesis of 2-[4-(1,3-dithian-2-yl)butoxy]oxane Cas no. 111741-88-9
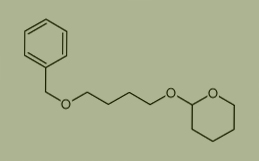
Used for the synthesis of 2-(4-phenylmethoxybutoxy)oxane
Cas no. 105966-45-8
Used for the synthesis of 3-bromotetrahydro-2H-pyran-2-carbonitrile
,Cas no. 1051940-71-6

Besides Safety Information of 3,4-Dihydro-2H-pyran CAS NO.110-87-2 is also important when handling it
|
Hazard Codes |
Xi |
|
WGK Germany |
3 |
|
H.S.Code: |
2932 9990.99 |
|
TSCA |
Yes |
|
HazardClass |
IRRITANT |
What is the appearance of 3,4-Dihydro-2H-pyran CAS NO.110-87-2? Please see the picture of 3,4-Dihydro-2H-pyran CAS NO.110-87-2, below
If you need the products .Please send your inquiry to us through e-mail: sales@yuansu-reagent.com
Specification of 3,4-Dihydro-2H-pyran CAS NO.110-87-2, is below
Apperance: Colorless to Light yellow clear liquid
Assay: 98 min by GC
IR identity: conform
Water:0.5% max by K.F.
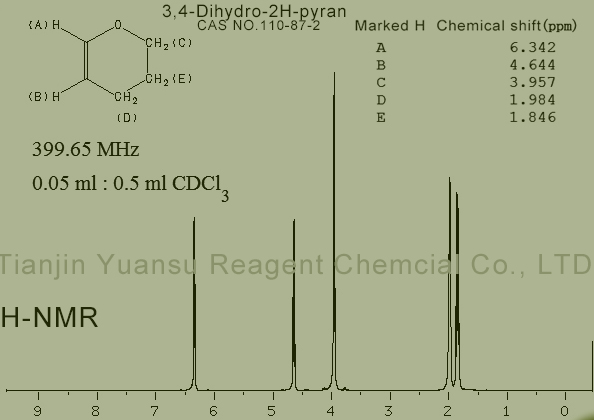
HNMR picture of 3,4-Dihydro-2H-pyran CAS NO.110-87-2 is as follows,
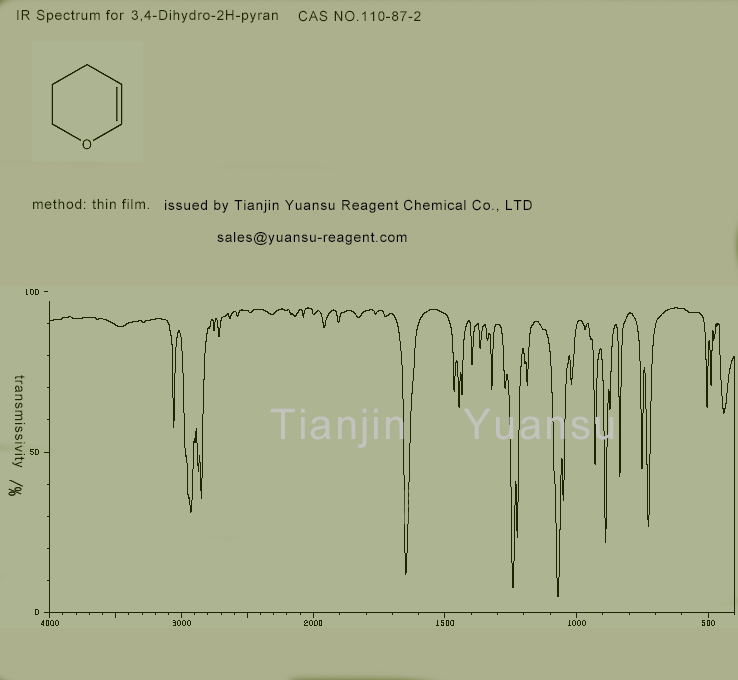
IR picture of 3,4-Dihydro-2H-pyran CAS NO.110-87-2 is as follows,
Reference of Article cited for your reference below,
(1)
A kind of preparation method of criborole
Publication Number: CN-115057877-A
Priority Date: 2022-07-01
(2)
A kind of method for synthesizing chiral 3,4-dihydro-2H-pyran compounds in water medium
Publication Number: CN-114835694-A
Priority Date: 2022-05-25