5-bromodihydroindole
What is 5-bromodihydroindole, cas no. 22190-33-6,a producer telling you the result.
CAS NO. 22190-33-6
If you need the products .Please send your inquiry to us through e-mail: sales@yuansu-reagent.com
To begin with, let us tell you what is the basic information of 5-bromodihydroindole CAS NO. 22190-33-6 ?
|
Flash Point |
124.7±24.3 °C |
Molecular Weight |
198.060 |
|
Density |
1.5±0.1 g/cm3 |
Molecular Formula |
C8H8BrN |
|
Boiling Point |
282.5±29.0 °C at 760 mmHg |
Melting Point |
36-40 °C(lit.) |
Like many stuff, it has many synonyms as follows
|
MFCD00027410 |
|
6-bromo-dihydroindole |
|
5-bromodihydroindole |
|
5-Brom-2,3-dihydro-1H-indol |
|
5-Bromoindoline |
|
5-bromo-1H-indoline |
First, the chemical is very special, some technical indexes as below
If you need the products .Please send your inquiry to us through e-mail: sales@yuansu-reagent.com
Ecological data
Do not discharge materials into the surrounding environment without government permission
Molecular structure data
1. Molar refractive index: 44.85
2. Molar volume (cm3/mol): 130.7
3. Waiting for Zhang Biarong (90.2K): 336.7
4. Surface tension (dyne/cm): 44.0
5. Polarization rate (10-24cm3): 17.78
Calculate chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: None
6. Topological molecular polarity surface area 12
7. Number of heavy atoms: 10
8. Surface charge: 0
9. Complexity: 126
10. Number of isotopic atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Nature and stability
If used and stored according to specifications, it will not decompose
Avoid contact with oxides
Storage method
Keep the storage container sealed
Put it in a tight storage container and store it in a cool, dry place
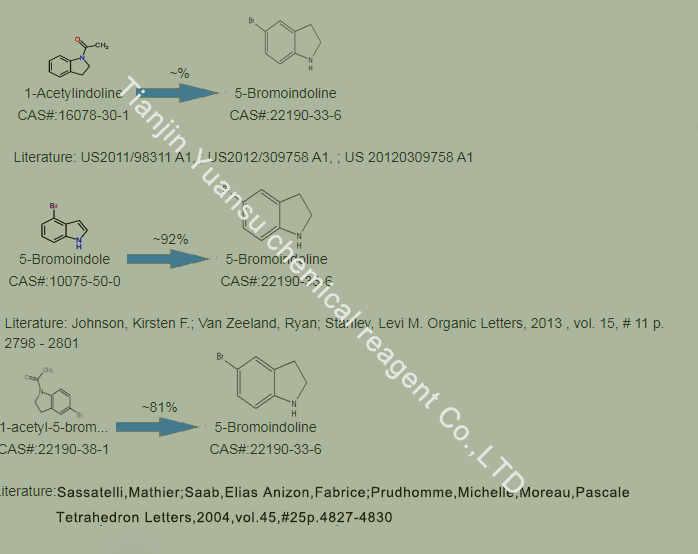
Second, the Synthetic Route we will recommend is the most important for your reference?
First, synthesis line of 5-bromodihydroindole CAS NO. 22190-33-6 as follows
The synthesis route can involve multiple steps. The following is a possible synthesis route, but please note that the specific synthesis method may vary depending on laboratory conditions and required purity:
A method for synthesizing 5-bromodihydroindole involves starting from N-formylindoline, followed by bromination reaction and subsequent hydrolysis step 1. The specific steps are as follows:
Dissolve N-formylindoline in a suitable solvent (such as dichloromethane).
Add dibromohydantoin to the solution and react at low temperature (such as -10~0 ° C) until the raw material disappears.
The reaction mixture is washed with sodium bisulfite aqueous solution, washed with water, concentrated and crystallized, and dried to obtain brominated intermediate products.
Dissolve the brominated intermediate obtained in the previous step in methanol, add potassium hydroxide aqueous solution, and heat the reaction until the raw material disappears.
The reaction mixture was quenched with water, concentrated, extracted with dichloromethane, crystallized and dried to obtain 5-bromodihydroindole.
Please note that the above synthesis route is only an example and may need to be adjusted according to specific laboratory conditions. In addition, due to the complex reaction conditions and potential hazards involved in chemical synthesis, safety operating procedures should be strictly followed in practical operations and carried out under the guidance of professional personnel.
Third, what is the usage of 5-bromodihydroindole CAS NO. 22190-33-6 ? pleas see below
If you need the products .Please send your inquiry to us through e-mail:sales@yuansu-reagent.com
Mainly used for
In the pharmaceutical field, 5-bromodihydroindole may be used as an active ingredient for the preparation of drugs or other pharmaceutical products. It may be used to treat various diseases or symptoms, although specific uses may involve patent protection or undisclosed information.
Scientific research: In scientific research, 5-bromodihydroindole can be used as an intermediate for synthesizing other chemical substances, or its physical and chemical properties can be studied under specific experimental conditions.
Industrial applications: Due to its unique chemical properties, 5-bromodihydroindole may also be used as a raw material or additive in industrial production, although specific applications may vary by industry.
Other usages as below
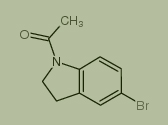
Used for the synthesis of 1-(5-bromo-2,3-dihydroindol-1-yl)ethanone
Cas no. 22190-38-1
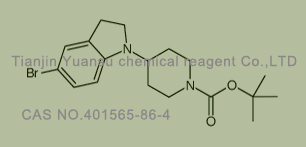
Used for the synthesis of tert-butyl 4-(5-bromo-2,3-dihydroindol-1-yl)piperidine-1-carboxylate
Cas no. 401565-86-4
Used for the synthesis of GSK2606414
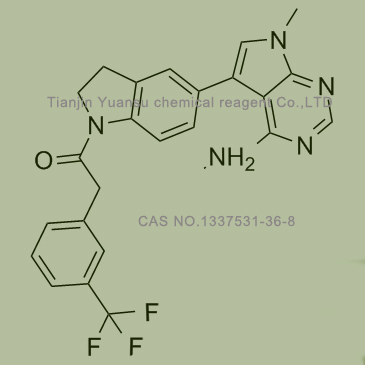
,Cas no. 1337531-36-8
Besides Safety Information of 5-bromodihydroindole CAS NO.22190-33-6 is also important when handling it
|
Hazard Codes |
Xi |
|
WGK Germany |
3 |
|
H.S.Code: |
2933990090 |
|
TSCA |
Yes |
|
HazardClass |
IRRITANT |
What is the appearance of 5-bromodihydroindole CAS NO.22190-33-6? Please see the picture of 5-bromodihydroindole CAS NO.22190-33-6, below
If you need the products .Please send your inquiry to us through e-mail: sales@yuansu-reagent.com
Specification of 5-bromodihydroindole CAS NO.22190-33-6, is below
Apperance: White to brown low melting point solid
Assay: 98 min by GC
IR identity: conform
Water:0.5% max by K.F.
Melting point: 36-40 °C
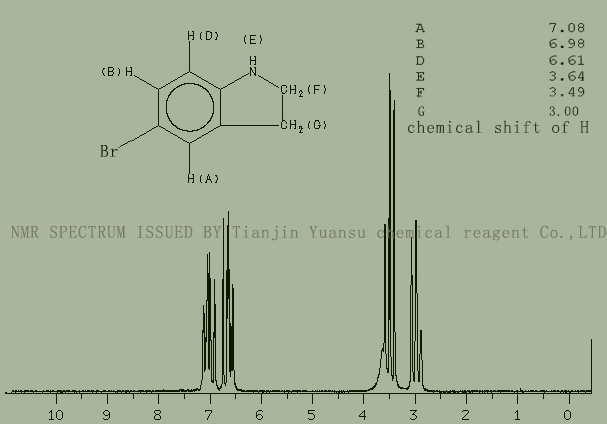
HNMR picture of 5-bromodihydroindole CAS NO.22190-33-6 is as follows,
Reference of Article cited for your reference below,
(1)
Synthesis and study of derivatives of 5-bromo-, 6-nitro-, and 5-bromo-6-nitro-1-glycosylisatins
Publication Date: 1985
Publication Name: Pharmaceutical Chemistry Journal
(2)
Synthesis of new 5-bromo derivatives of indole and spiroindole phytoalexins
Publication Date: 2015
Publication Name: Chemical Papers
(3)
An Expeditious One-Pot Synthesis of N-Substituted 6-Nitroindoles from Indolines
Publication Date: 2003
Publication Name: Monatshefte für Chemie / Chemical Monthly