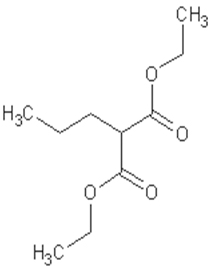
Diethyl propylmalonate
CAS NO. 133-13-1
If you need the products .Please send your inquiry to us through e-mail: info@pewax.com.cn
To begin with, let us tell you what is the basic information of Diethyl propylmalonate CAS NO. 2163-48-6?
|
Flash Point |
91.7±0.0 °C |
Molecular Weight |
202.247 |
|
Density |
1.0±0.1 g/cm3 |
Boiling Point |
221.5±0.0 °C at 760 mmHg |
|
Molecular Formula |
C10H18O4 |
MDL No. |
MFCD00009168 |
Like many stuff, it has many synonyms as follows
|
Propanedioic acid, propyl-, diethyl ester |
|
Propanedioic acid, 2-propyl-, diethyl ester |
|
Malonic acid, propyl-, diethyl ester |
|
Malonic acid,propyl-,diethyl ester |
|
Propylmalonic acid diethyl ester |
|
Diethyl 2-propylmalonate |
|
Diethyl propylmalonate |
|
DIETHYL N-PROPYLMALONATE |
First, the chemical is very special, some technical indexes as below
Ecological data
It is slightly harmful to water. Do not let undiluted or large amounts of products come into contact with groundwater, waterways, or sewage systems. Without government permission, do not discharge materials into the surrounding environment
Molecular structure data
1. Molar refractive index: 51.88
2. Molar volume (cm3/mol): 200.9
3. Isometric volume (90.2K): 476.8
4. Surface tension (dyne/cm): 31.7
5. Polarization rate (10-24cm3): 20.56
Computational Chemistry Data
1. Reference value for hydrophobic parameter calculation (XlogP): 2.3
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond receptors: 4
4. Number of rotatable chemical bonds: 8
5. Number of tautomers: None
6. Topological molecule polarity surface area 52.6
7. Number of heavy atoms: 14
8. Surface charge: 0
9. Complexity: 171
10. Isotope atomic number: 0
11. Determine the number of atomic structure centers: 0
12. Number of uncertain atomic structure centers: 0
13. Determine the number of chemical bond stereocenters: 0
14. Uncertain number of chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and Stability
Avoiding oxides, hazardous decomposition products include carbon monoxide and carbon dioxide
Storage method
The storage area must be away from oxidants, keep the container sealed, place it in a tight container, and store it in a cool, dry place
Second, the Synthetic Route we will recommend is the most important for your reference?
First, synthesis line of Diethyl propylmalonate CAS NO. 2163-48-6 as follows
If you need the products .Please send your inquiry to us through e-mail: info@pewax.com.cn

The preparation of diethyl propylmalonate is usually carried out through esterification reaction. The specific step is to esterify diethyl malonate anhydride with ethanol to generate diethyl malonate.
Third, what is the usage of Diethyl propylmalonate CAS NO. 2163-48-6 ? pleas see below
If you need the products .Please send your inquiry to us through e-mail: info@pewax.com.cn
Mainly used for the intermediates for organic synthesis and pharmaceutical industry and pesticide industry.
Diethyl propylmalonate is mainly used as a solvent and plasticizer, and can be used in the rubber, resin, and coating industries.
Besides Safety Information of Diethyl propylmalonate CAS NO. 2163-48-6 is also important when handling it
|
Hazard Codes |
Xi |
|
WGK Germany |
3 |
|
H.S.Code: |
2917190090 |
|
TSCA |
Yes |
|
HazardClass |
IRRITANT |
What is the appearance of Diethyl propylmalonate CAS NO. 2163-48-6? Please see the picture of Diethyl propylmalonate CAS NO. 2163-48-6, below
If you need the products .Please send your inquiry to us through e-mail: info@pewax.com.cn
Specification of Diethyl propylmalonate CAS NO. 2163-48-6, is below
Apperance: colorless clear liquid
Assay: 99 % min by GC
IR identity: conform
Water:0.5% max by K.F.
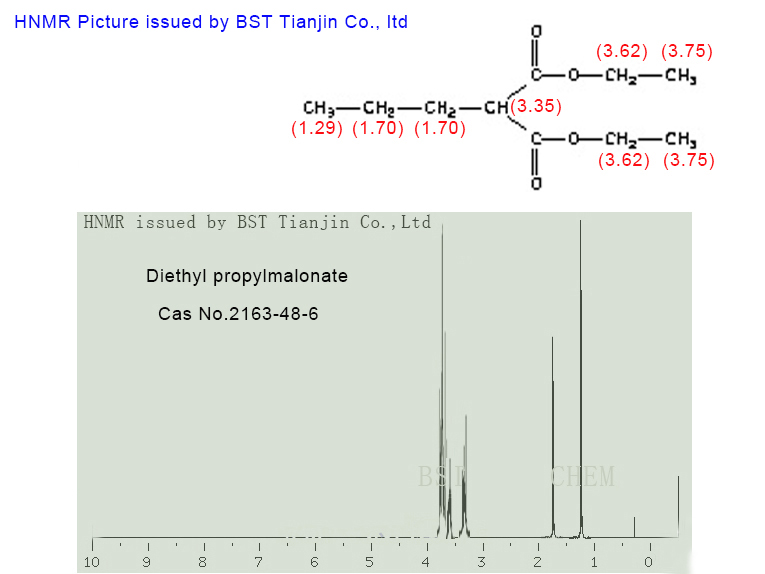
HNMR picture of Diethyl propylmalonate CAS NO. 2163-48-6 is as follows,
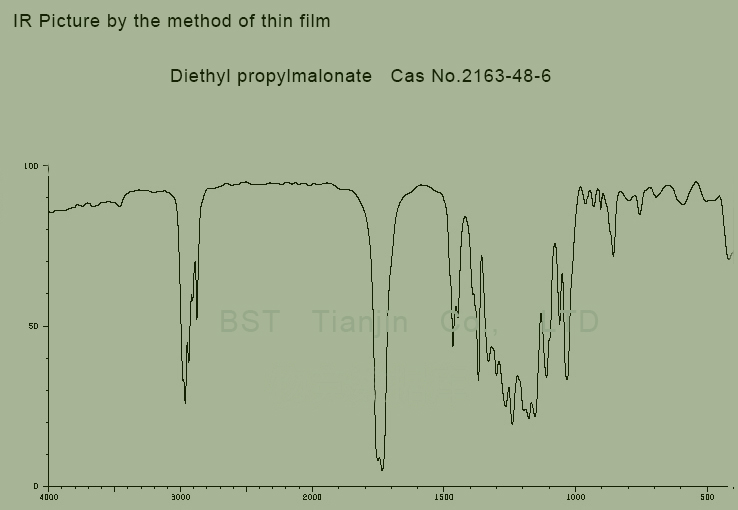
IR picture of Diethyl propylmalonate CAS NO. 2163-48-6 is as follows,
Reference of Article cited for your reference below,
(1)
An atom counting and electrophilicity based QSTR approach
Publication Name: Journal of Chemical Sciences
Publication Date: 2007-09
DOI: 10.1007/s12039-007-0061-1
(2)
Microparticle Depots for Controlled and Sustained Release of Endosomolytic Nanoparticles
Publication Date: 2019
Publication Name: Cellular and Molecular Bioengineering
(3)
Controlled Endolysosomal Release of Agents by pH-responsive Polymer Blend Particles
PMID: 25592550
Publication Date: 2015
Publication Name: Pharmaceutical Research