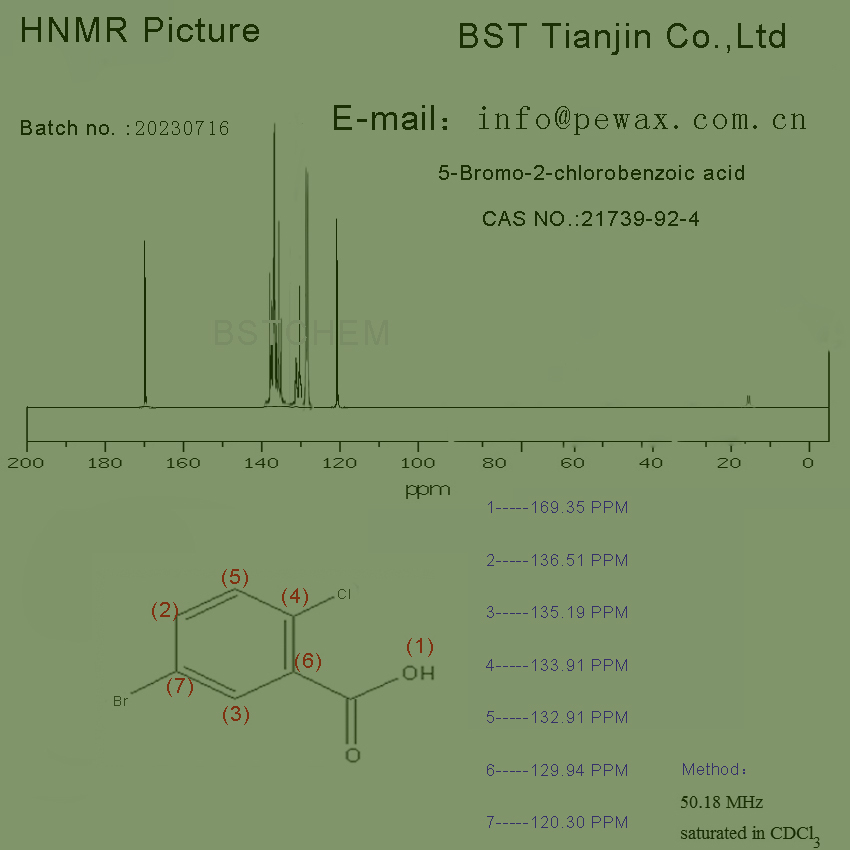
5-Bromo-2-chlorobenzoic acid
CAS NO. 21739-92-4
If you need the products .Please send your inquiry to us through e-mail: info@pewax.com.cn
To begin with, let us tell you what is the basic information of 5-Bromo-2-chlorobenzoic acid Cas No. 21739-92-4 ?
|
Molecular Formula |
C7H4BrClO2 |
Molecular Weight |
235.462 |
|
Density |
1.8±0.1 g/cm3 |
Boiling Point |
324.5±27.0 °C at 760 mmHg |
|
Exact Mass |
233.908310 |
Melting Point |
154-156 °C(lit.) |
|
MDL No |
MFCD00002415 |
Flash Point |
150.1±23.7 °C |
Like many stuff, it has many synonyms as follows
|
EINECS 244-558-5 |
|
Benzoic acid,5-bromo-2-chloro |
|
5-bromo-2-chloro benzoic acid |
|
5-Brom-2-chlor-benzoesaeure |
|
Benzoic acid, 5-bromo-2-chloro- |
| QVR BG EE |
The chemical is very special
If you need the products .Please send your inquiry to us through e-mail:info@pewax.com.cn
Molecular structure data
1. Molar refractive index: 45.76
2. Molar volume (m3/mol): 130.0
3. Isometric volume (90.2K): 355.7
4. Surface tension (dyne/cm): 55.9
5. Polarization rate (10-24cm3): 18.14
Computational Chemistry Data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond receptors: 2
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: None
6. Topological molecule polarity surface area 37.3
7. Number of heavy atoms: 11
8. Surface charge: 0
9. Complexity: 163
10. Number of Isotope Atoms: 0
11. Determine the number of atomic structure centers: 0
12. Number of uncertain atomic structure centers: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Second, the Synthetic Route we will recommend is the most important for your reference?
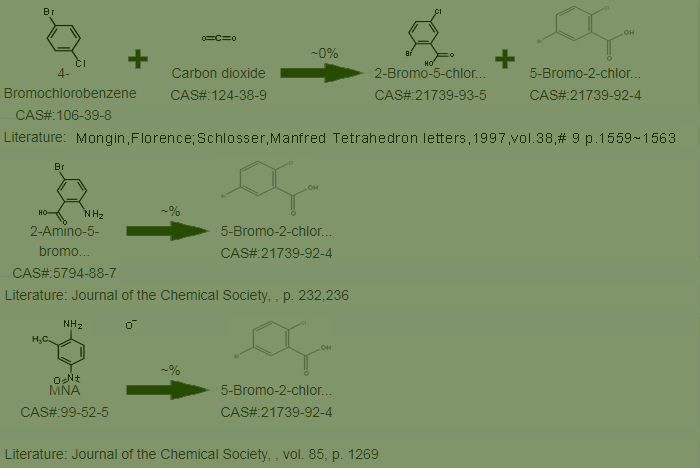
First, synthesis line of 2-chloro-5-bromobenzoic acid ,Cas No.: 21739-92-4 ,as follows
Using 5-bromo-2-chlorobenzyl alcohol as the raw material, oxidation was carried out in a mixed solvent of Pyridine Chlorochrome/CH2Cl to form
If you need the products .Please send your inquiry to us through e-mail: info@pewax.com.cn
Second,synthesis line of 5-Bromo-2-chlorobenzoic acid Cas No.: 21739-92-4 ,as follows
2-Chlorobenzoic acid was monobrominated using NBS (N-bromosuccinimide)/sulfuric acid system, which improved the selectivity of the raw material and reduced the cost. The ratio of 5-bromo-2-chlorobenzoic acid and 3-bromo-2-chlorobenzoic acid produced was increased to 18:1, and water was directly added for crystallization and purification during post-treatment. The organic solvent extraction process was omitted, and a single solvent was used for recrystallization. This not only simplifies the process flow, but also facilitates the recovery and application of solvents. The purity of the obtained 5-bromo-2-chlorobenzoic acid is>99%, and the yield can reach 80%.
2-Chlorotrichlorotoluene is used as the raw material to react with a bromination reagent under the catalysis of the first catalyst to generate 2-chloro-5-bromotrichotoluene. Water is added to the reaction, and the catalyst is hydrolyzed. After post-treatment, 5-bromo-2-chlorobenzoic acid is purified; The first catalyst is made by compounding iron bromide, ferrous bromide, copper bromide, (ferrocene methyl) trimethylammonium bromide, and ferrocene bromide.
Other synthesis lines of 5-Bromo-2-chlorobenzoic acid Cas No.: 21739-92-4 as follows?
Third, what is the usage of 5-Bromo-2-chlorobenzoic acid Cas No.: 21739-92-4 ? pleas see below
Main Usage:
5-bromo-2-chlorobenzoic acid is a common key intermediate for the synthesis of a new anti diabetes drug SGLT-2 inhibitor. Therefore, it is particularly important to find a suitable preparation method of 5-bromo-2-chlorobenzoic acid.
SGLT-2 inhibitor is a new generation of diabetes drugs. It controls blood sugar by inhibiting the reabsorption of glucose in the kidney. Its mechanism of action is unique and does not depend on β The degree of cellular dysfunction or insulin resistance will not vary with the effect β The decline in cellular function due to failure or severe insulin resistance does not result in adverse reactions caused by traditional drugs, and has multiple clinical advantages, especially in clinical practice, demonstrating the advantage of reducing cardiovascular risk.
If you need the products .Please send your inquiry to us through e-mail: info@pewax.com.cn
Other usages as below
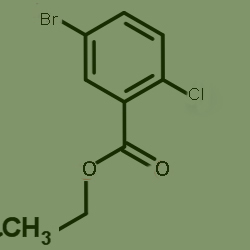
One kind of raw material for producing ethyl 5-bromo-2-chlorobenzoate, cas no 76008-73-6,which is as follows
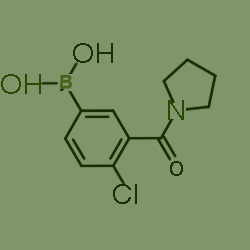
One kind of raw material for producing (4-Chloro-3-(pyrrolidine-1-carbonyl)phenyl)boronic acid, cas no 871332-75-1,which is as follows
Besides Safety Information of 5-Bromo-2-chlorobenzoic acid Cas No.: 21739-92-4 is also important when handling it
|
Hazard Codes |
Xi |
|
H.S. Code |
2933 9900.99 |
What is the appearance of 5-Bromo-2-chlorobenzoic acid Cas No.: 21739-92-4 ? Please see the picture of (S)-2-Benzylsuccinic acid Cas No.: 3972-36-9 below
If you need the products .Please send your inquiry to us through e-mail: info@pewax.com.cn
Specification of 5-Bromo-2-chlorobenzoic acid Cas No.: 21739-92-4 is below
Apperance: white to light yellow crystal powder Assay: 99 % min by HPLC
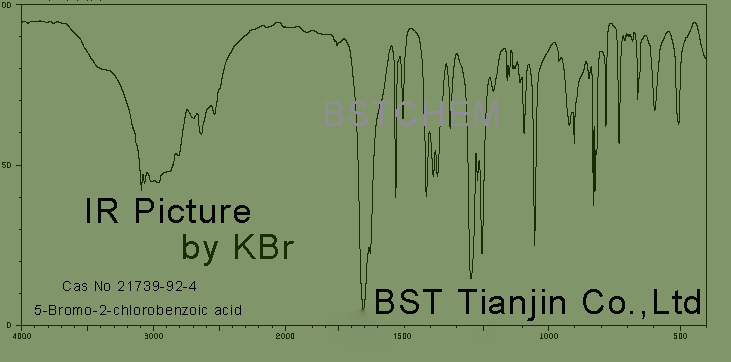
IR identity: conform
Melting Point:154~156℃
Water by KF:0.5% max.

HNMR picture of 5-Bromo-2-chlorobenzoic acid ,Cas No.: 21739-92-4 is as follows,
IR picture of 5-Bromo-2-chlorobenzoic acid ,Cas No.: 21739-92-4 is as follows,
Reference of Article cited for your reference below,
(1)
Primary qHTS Assay for Foot and Mouth Disease Virus Antivirals against NCGC Sytravon and CBC Compound Libraries: Firefly Luciferase Signal
Activity:
Activity Type: Potency
Activity Value: 44.6684 µM
BioAssay AID: 1745856
Substance SID: 377647366
Compound CID: 33127
(2)
Publication Name: Journal of Structural Chemistry
Publication Date: 2020-07
DOI: 10.1134/s0022476620070215
(3)
Copper-Catalyzed Cyclization of 2-Halobenzoic Acids with Benzene-1,2-diamine
Publication Name: Science of Synthesis
Publication Date: 2013