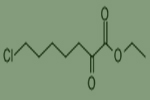
Ethyl 7-chloro-2-oxoheptanoate
CAS NO. 78834-75-0
If you need the products .Please send your inquiry to us through e-mail: info@pewax.com.cn
To begin with, let us tell you what is the basic information of Ethyl 7-chloro-2-oxoheptanoate Cas No. 78834-75-0 ?
|
Molecular Weight |
206.66700 |
Molecular Formula |
C9H15ClO3 |
|
Density |
1.093 g/cm3 |
Boiling Point |
281.2ºC at 760 mmHg |
|
LogP |
1.91780 |
CAS Number |
78834-75-0 |
|
MDL No. |
MFCD07698675 |
Flash Point |
111.3ºC |
Like many stuff, it has many synonyms as follows
|
EINECS 278-992-1 |
|
ethyl 7-chloro-2-oxoheptanoate |
The chemical is very special
If you need the products .Please send your inquiry to us through e-mail: info@pewax.com.cn
Computational Chemistry Data
1. Reference value for hydrophobic parameter calculation (XlogP): 2
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond receptors: 3
4. Number of rotatable chemical bonds: 8
5. Number of tautomers: 2
6. Topological molecule polarity surface area: 43.4
7. Number of heavy atoms: 13
8. Surface charge: 0
9. Complexity: 168
10. Number of Isotope Atoms: 0
11. Determine the number of atomic structure centers: 0
12. Number of uncertain atomic structure centers: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Second, the Synthetic Route we will recommend is the most important for your reference?

First, synthesis line of Ethyl 7-chloro-2-oxoheptanoate Cas No. 78834-75-0 ,as follows
If you need the products .Please send your inquiry to us through e-mail: info@pewax.com.cn
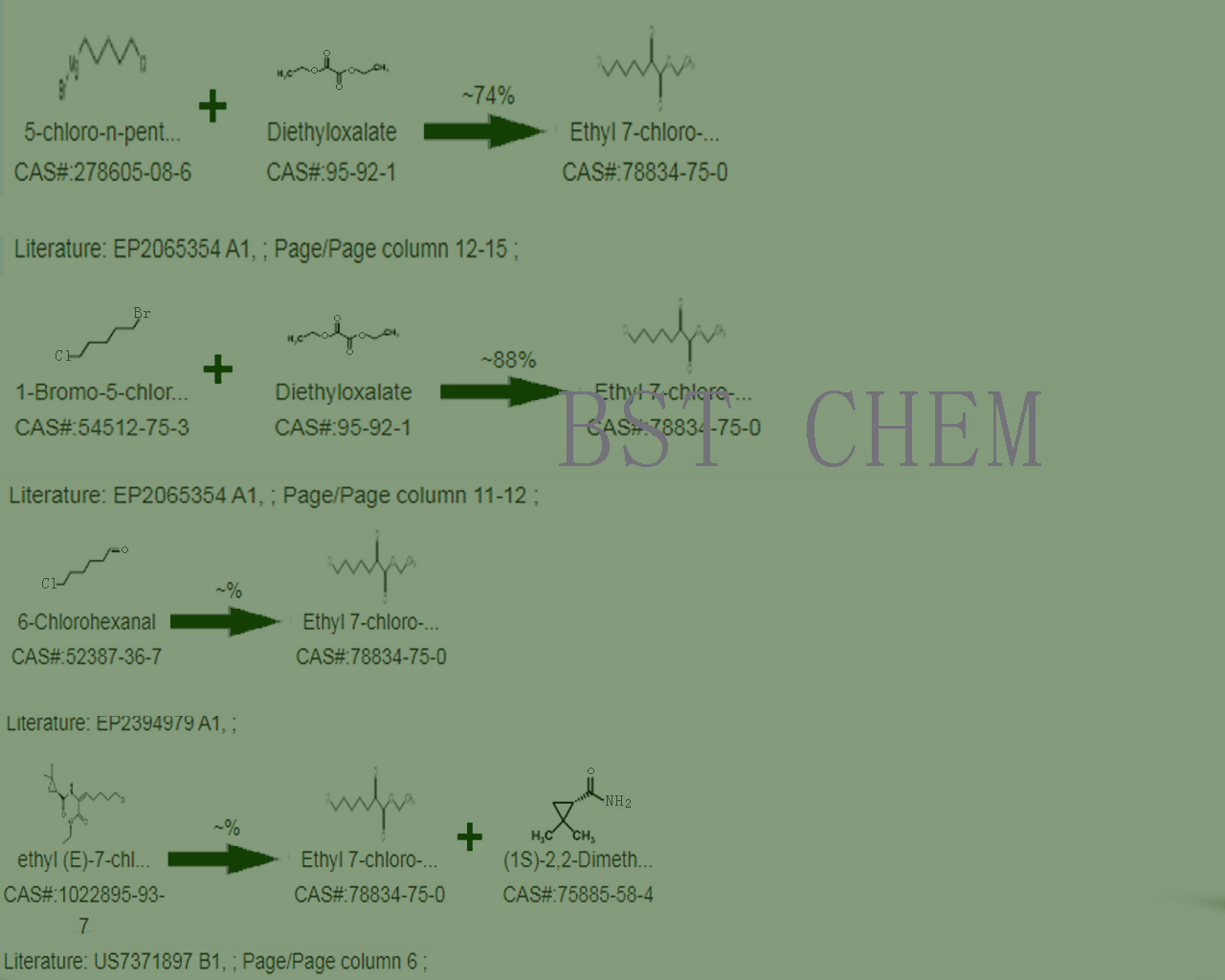
Ethyl 7-chloro-2-oxoheptoate was synthesized from 1-bromo-5-chloropentane and diethyl acetate in a yield of approximately 88%;
Synthesis line in lab as below
Step 1: Under dry N2 protection, burn in a four port furnace equipped with a condensing tube, thermometer, stirrer, and 50ml constant pressure drip funnel .Add a mixture of 7.3g of diethyl oxalate, 9.3g of 1-bromo-5-chloropentane, and 30ml of tetrahydrofuran to the bottle;
Step 2: Quickly add 1.0g PSIM MgBr under the condition of -25 ℃, and maintain the reaction temperature controlled at around -10 ℃ during the drop addition reaction. After the drop addition is completed, continue the reaction for 1 hour;
Step 3: Hydrolyze with 15ml of 4M hydrochloric acid, and ensure that the reaction temperature is below 0 ℃ during the acid hydrolysis process;Separate the organic layer, extract the water layer with dichloromethane, wash with saturated sodium chloride solution, and add NaHS03 saturated solution for oscillation;
Step 4: After shaking, the organic layer and water layer are neutralized with a saturated NaHC03 solution. The organic layer is rotated and evaporated to produce tetrahydrofuran. The organic layer is merged and neutralized with saturated NaHC03, washed with NaHS03, and then washed with NaCl until neutral. The solvent is evaporated and reduced pressure distillation is carried out to obtain 7-chloro-2-oxoheptate ethyl ester.
Second,synthesis line of Ethyl 7-chloro-2-oxoheptanoate Cas No. 78834-75-0 ,as follows
Synthesis of 7-chloro-2-oxoheptoate ethyl ester using diethyl acetate in a yield of approximately 74%;
Other synthesis lines of Ethyl 7-chloro-2-oxoheptanoate Cas No. 78834-75-0 as follows?
Third, what is the usage of Ethyl 7-chloro-2-oxoheptanoate Cas No. 78834-75-0? pleas see below
Main Usage:
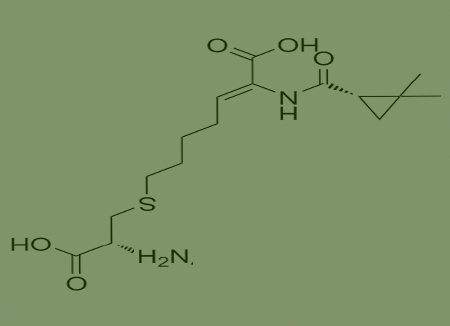
Ethyl 7-chloro-2-oxoheptoate is an important starting material for the synthesis of Cilastatin. Silastatin is a kidney dehydrogenase inhibitor developed by an American company. It can inhibit the renal metabolism of the broad-spectrum antibacterial drug imipenem, which is currently used in clinical practice. While reducing renal toxicity, it can also increase the blood antibacterial concentration of imipenem, with significant therapeutic effects. Therefore, in the past decade, domestic and foreign chemists have conducted extensive research on how to improve the purity and yield of synthesized silastatin. Due to the fact that ethyl 7-chloro-2-oxoheptoate is an important starting material for the synthesis of cilastatin, and the purity of the raw material is an important factor affecting the product cilastatin, obtaining high-purity and high-yield ethyl 7-chloro-2-oxoheptoate has become the key to improving the purity of cilastatin and reducing the production cost of the drug.
If you need the products .Please send your inquiry to us through e-mail: info@pewax.com.cn
Other usages as below
One kind of raw material for producing cilastatin cas no 82009-34-5,which is as follows
Besides Safety Information of Ethyl 7-chloro-2-oxoheptanoate Cas No. 78834-75-0 is also important when handling it
|
Hazard Codes |
Xi |
|
H.S. Code |
2918300090 |
What is the appearance of Ethyl 7-chloro-2-oxoheptanoate Cas No. 78834-75-0 ? Please see the picture of Ethyl 7-chloro-2-oxoheptanoate Cas No. 78834-75-0 below
If you need the products .Please send your inquiry to us through e-mail: info@pewax.com.cn
Specification of Ethyl 7-chloro-2-oxoheptanoate Cas No. 78834-75-0 is below
Apperance:yellow oil liquid
Assay: 98 % min by GC
IR identity: conform
Water by KF:0.5% max.
HNMR picture of Ethyl 7-chloro-2-oxoheptanoate Cas No. 78834-75-0 is as follows,
Reference of Article cited for your reference below,
(1)Ethyl 7-chloro-2-oxoheptanoate
Publication Name: ACS Chemical Biology
Publication Date: 2020-05-27
PMID: 32459468
DOI: 10.1021/acschembio.0c00343
Publication Name: Antiviral Research
Publication Date: 2020-01
PMID: 31786250