Methoxycarbonyl-L-tert-leucine
CAS NO. 162537-11-3
If you need the products .Please send your inquiry to us through e-mail: info@pewax.com.cn
To begin with, let us tell you what is the basic information of Methoxycarbonyl-L-tert-leucine Cas No. 162537-11-3?
|
Vapour Pressure |
0.0±1.5 mmHg at 25°C |
Molecular Weight |
189.209 |
|
Density |
1.1±0.1 g/cm3 |
CAS Number |
162537-11-3 |
|
Molecular Formula |
C8H15NO4 |
Melting Point |
109ºC |
|
Flash Point |
147.9±23.2 °C |
Boiling Point |
320.9±25.0 °C at 760 mmHg |
Like many stuff, it has many synonyms as follows
N-Methoxycarbonyl-L-tert-leucine
N-(Methoxycarbonyl)-L-tert-leucine
N-(Methoxycarbonyl)-3-methyl-L-valine
(2S)-2-(methoxycarbonylamino)-3,3-dimethylbutanoic acid
L-Valine, N-(methoxycarbonyl)-3-methyl-
Methoxycarbonyl-L-tert-leucine
(S)-2-((Methoxycarbonyl)amino)-3,3-dimethylbutanoic acid
The chemical is very special

If you need the products .Please send your inquiry to us through e-mail: info@pewax.com.cn
Second, the Synthetic Route we will recommend is the most important for your reference?
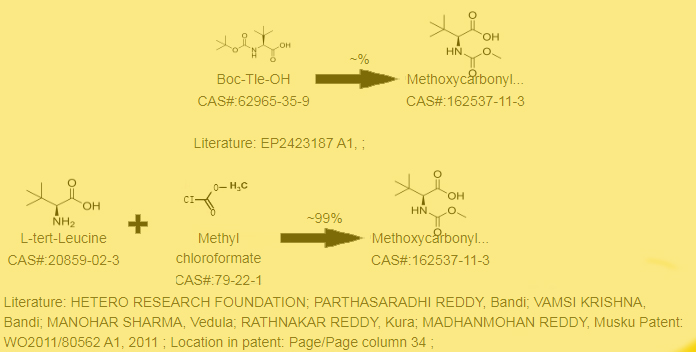
Synthesis line of Methoxycarbonyl-L-tert-leucine Cas No.: 162537-11-3 as follows

If you need the products .Please send your inquiry to us through e-mail: info@pewax.com.cn
The key to the preparation of N-methoxycarbonyl-L-tert leucine is to obtain chiral pure L-tert leucine. There are two methods for preparing L-tert leucine: resolution method and asymmetric synthesis method [3]. The resolution agents used in the chemical resolution method include diphenylphthalein tartaric acid, quinine, or quinidine. Biological enzymes, due to their high selectivity, can also be used for resolution, but only L-type tert leucine can be obtained. The method described has a common drawback of preparing racemic tert leucine first, derivatizing it, and then using corresponding resolution agents or enzymes for resolution. It is relatively expensive and often has low purity, and the yield of a single configuration prepared by the resolution method is generally less than 50% [4]. Chiral asymmetric synthesis is a relatively direct and purposeful method for synthesizing chiral target compounds. Tertiary leucine belongs to a category of a-amino acids, and for a-amino acids, the classic chemical synthesis method is the Strecker reaction. The stereochemistry of the product is constructed by asymmetric addition of cyano to imine, and then a-amino acids are prepared through hydrolysis and other reactions. During the reaction process, the addition of ligands or Lewis acids can achieve high selectivity [5]. However, the disadvantage of the Slrecker reaction is that it is not easy to achieve mass production and preparation, and the toxicity of cyanide groups is also relatively high, causing serious environmental pollution [6]. The preparation of chiral amino acid compounds by enzymatic transformation not only has mild reaction conditions, high yield and optical purity, but also avoids pollution problems caused by chemical synthesis methods. It has great application potential and broad development prospects.
Synthesis line of (2S)-2-(methoxycarbonylamino)-3,3-dimethylbutanoic acid Cas No.: 162537-11-3 No.2
(1) Add Na2CO3 (8.9 g, 84 mmol) to a 1N NaOH (168 mL) solution of L-alanine (8 g, 168 mmol). Cool the solution to 0 ° C and add methyl chloroformate (20.5 mL, 172 mm) dropwise
(2) Add Na2CO3 (276 mg, 2.6 mmol) to an aq solution of NaOH (5 mL 1.00 M solution, 5 mmol) and l tert leucine (656 mg, 5 mmol), and cool the resulting solution in an ice water bath. Add methyl chloroformate (0.42 mL, 5.4 mmol) dropwise to the mixture, remove the cooling bath, and stir at room temperature for 3.25 hours. The reaction mixture was washed with ether (3x9 mL), the aqueous phase was cooled in an ice water bath, acidified with conc HCl to a pH of 1-2, and extracted with CH2Cl2 (3x9 mL). Dry the organic phase with magnesium sulfate, filter, and vacuum concentrate to obtain an oily liquid. Then the oil and toluene (3x9 mL) azeotrope and finally dry under high vacuum. Obtain a white solid (670 mg, 71%). (CDCl3, δ= 7.26 ppm, 500 MHz): 9.57 (br s, 1H), 5.31 (d, 1H), 4.20 (d, 1H), 3.70 (s, 3H), 1.03 (s, 9H)
(3) In an aqueous solution (60 mL) of D-phenylglycine (10.0 g, 66.1 mmol) and NaOH (21.2 g, 265 mmol) at 0 ° C, methyl chloroformate (10.2 mL, 133 mmol) was added dropwise for 20 minutes. Stir the obtained reaction at 0 ° C for 1 hour, and then acidify with concentrated hydrochloric acid (25 mL, 300 mmol). Using ethyl acetate (3 × Extract an acidic solution of 100 mL, dry the organic compound on MgSO4, filter, vacuum concentrate, and provide the compound without further purification to directly obtain N-methoxycarbonyl-L-tert leucine.
(4)23.5 ml of methyl chloroformate (2S) -2- [(methoxycarbonyl) amino] -3,3-dimethylbutyric acid was added to a solution of 20 g of 2 (S) - amino 3,3-dimethylbutyric acid, and a mixture of 252 ml of 2N sodium hydroxide aqueous solution and 80 ml of dioxane was added. The reaction time was 20 minutes, and the reaction solution was heated at 60 ° C for 14 hours. Cool it to room temperature and wash twice with dichloromethane. Acidify the aqueous phase with 4N hydrochloric acid to pH 2, and extract three times with ethyl acetate. Combine, dry (Na2SO4), evaporate and concentrate the organic extract, and the product begins to solidify. The solid was digested with n-hexane to obtain a white powder of (2S) -2- [(methoxycarbonyl) amino] -3,3-dimethylbutyric acid. Melting point: 106-108 ° C.
Other synthesis lines of (2S)-2-(methoxycarbonylamino)-3,3-dimethylbutanoic acid as follows?
Third, what is the usage of N-(Methoxycarbonyl)-3-methyl-L-valine Cas No.: 162537-11-3 ? pleas see below
Main Usage:
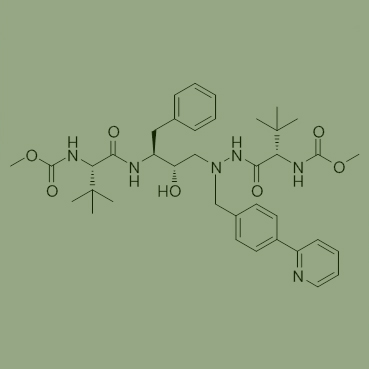
Used as one Intermediates of Atazanavir (ATV) for AIDS Cas No. 198904-31-3
If you need the products .Please send your inquiry to us through e-mail: info@pewax.com.cn
Other usages as below
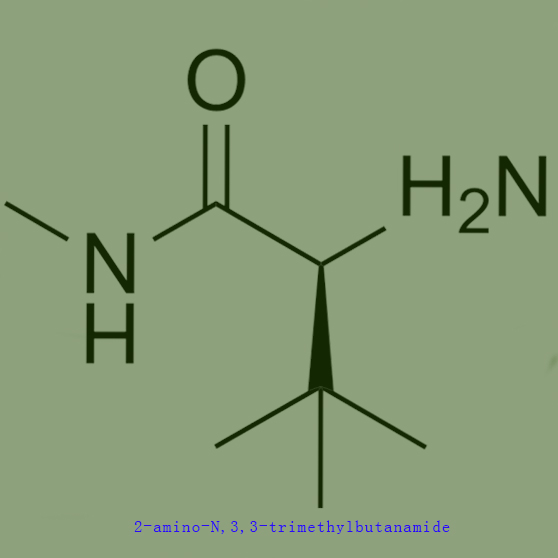
One kind of raw material for producing 2-amino-N,3,3-trimethylbutanamide cas no 89226-12-0,which is as follows
Besides Safety Information is also important when handling it
|
Hazard Codes |
Xi |
|
RIDADR |
NONH for all modes of transport |
|
HS Code |
2924199090 |
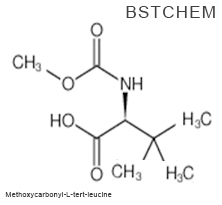
What is the appearance of N-(Methoxycarbonyl)-3-methyl-L-valine? Please see the picture of Methoxycarbonyl-L-tert-leucine Cas No.: 162537-11-3 below
If you need the products .Please send your inquiry to us through e-mail: info@pewax.com.cn
Specification of N-(Methoxycarbonyl)-3-methyl-L-valine Cas No.: 162537-11-3 is below
Apperance: white or off-white crytal solid
Assay: 99 % min by HPLC
IR identity: conform
Melting Point:109~113℃
Water by KF:0.5% max.

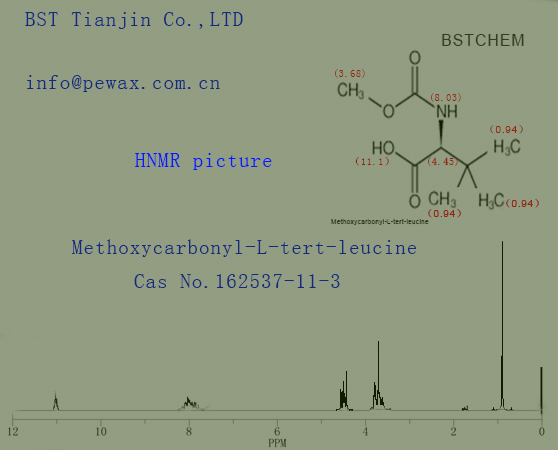
HNMR picture of N-(Methoxycarbonyl)-3-methyl-L-valine Cas No.: 162537-11-3 is as follows,
Reference of Article cited for your reference below,