2-Butoxyethyl acetate Cas no. 112-07-2
If you want to buy the chemical ,please inquiry us through E-mail: chem@pewax.com.cn
First of all,
What is the basic information of the chemical 2-Butoxyethyl acetate?
|
Vapour Pressure |
0.5±0.3 mmHg at 25°C |
Molecular Weight |
160.211 |
|
Density |
0.9±0.1 g/cm3 |
Flash Point |
76.1±0.0 °C |
|
Molecular Formula |
C8H16O3 |
Melting Point |
-63°C |
|
Boiling Point |
192.0±0.0 °C at 760 mmHg |
CAS Number |
112-07-2 |
Second ,what is its MSDS that can show you how to handle it when using it ?
Whenever, safe is always in the first place,can you view the information below?
|
Hazard Codes |
Xn:Harmful; |
|
Risk Phrases |
R20/21 |
|
Safety Phrases |
S24 |
|
WGK Germany |
1 |
|
HS Code |
2909430000 |
|
Personal Protective Equipment |
Eyeshields;Faceshields;full-face respirator (US);Gloves;multi-purpose combination respirator cartridge (US);type ABEK (EN14387) respirator filter |
Properties and Stability of 2-Butoxyethyl acetate Cas no. 112-07-2
Avoid contact with oxides and alkalis.
Storage method of 2-Butoxyethyl acetate Cas no. 112-07-2
Store in a cool and ventilated warehouse. Keep away from sparks and heat sources. Prevent direct sunlight. Keep the container sealed. It should be stored separately from oxidants and acids, and mixed storage should be avoided. Equip corresponding types and quantities of fire-fighting equipment. The storage area should be equipped with emergency response equipment for leaks and suitable storage materials
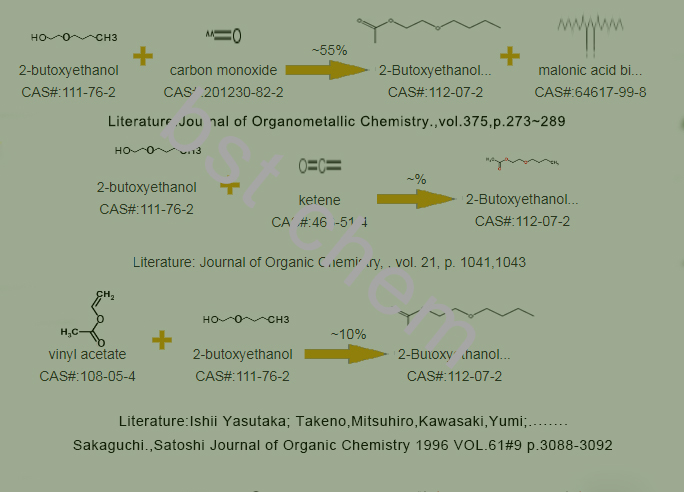
Third, how we can produce 2-Butoxyethyl acetate Cas no. 112-07-2 for your reference when you synthesis it in the future, are you interested in the good content?
If you want to buy the chemical ,please inquiry us through E-mail: chem@pewax.com.cn
One of Synthetic lines of 2-Butoxyethyl acetate Cas no. 112-07-2 as below
filling a composite metal oxide catalyst into a fixed bed reactor, and using butyl acetate and ethylene oxide as raw materials through alkylation reaction under the catalysis of the composite metal oxide catalyst to obtain ethylene glycol butyl ether acetate. Therefore, the preparation of the composite metal oxide catalyst is of utmost importance in this method.
Preparation of composite metal oxide catalysts,
Firstly, a conventional co precipitation method is used to prepare composite metal hydroxides, and they are dried at 60-80 ℃ for 12-24 hours. Then, it is reacted with fatty acids to obtain a complex, which is then calcined below 500 ℃. Finally, the calcined complex is granulated to obtain a composite metal oxide catalyst.
After the preparation of the composite metal oxide catalyst, put a 1:1 to 5:1 ratio of butyl acetate to ethylene oxide was added to the fixed bed reactor, followed by the addition of 20 to 40 mesh particles of the composite metal oxide catalyst (with a mass space velocity of 2.5 to 4.1 h-1). Under the catalysis of a composite metal oxide catalyst, the two undergo an alkylation reaction. The reaction temperature during the alkylation reaction is 120~150 ℃, and the reaction pressure is 0.1~0.5MPa. Finally, the product after the reaction is cooled and separated into gas and liquid to obtain ethylene glycol butyl ether acetate.
Other Synthetic lines of 2-Butoxyethyl acetate Cas no. 112-07-2 as below
Besides, like other chemicals, 2-Butoxyethyl acetate Cas no. 112-07-2 has synonym,do you want to futher know?
|
EINECS 203-933-3 |
|
ethylene glycol butyl ether acetate |
|
Ethanol, 2-butoxy-, acetate |
|
GLYCOL MONOBUTYL ETHER ACETATE |
|
2-butoxy-ethanoacetate |
|
Acetic Acid 2-Butoxyethyl Ester |
|
2-butoxyethyl ethanoate |
|
Butoxyethanol acetate |
|
Butylglycol acetate |
|
2-Butoxyethyl acetate |
|
Butylcelosolvacetat |
|
Ethylene glycol mono-n-butyl ether acetate |
|
n-Butoxyethanol acetate |
|
2-butoxyethanolacetate |
|
ektasolveebacetate |
|
acetic acid-(2-butoxy-ethyl ester) |
|
2-Butoxyethanol acetate |
|
2-(n-butoxy)ethyl acetate
BUTOXYETHYL ACETATE |
Certainly ,do you concern about how you can identify the chemial is what you righ need or not?
If you want to buy the chemical ,please inquiry us through E-mail: chem@pewax.com.cn
Molecular structure data
1. Molar refractive index: 42.63
2. Molar volume (cm3/mol): 170.4
3. Isometric volume (90.2K): 394.8
4. Surface tension (dyne/cm): 28.8
5. Polarization rate (10-24cm3): 16.90
Computational Chemistry Data
1. Reference value for hydrophobic parameter calculation (XlogP): 1.2
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond receptors: 3
4. Number of rotatable chemical bonds: 7
5. Number of tautomers: None
6. Topological molecule polarity surface area 35.5
7. Number of heavy atoms: 11
8. Surface charge: 0
9. Complexity: 102
10. Number of Isotope Atoms: 0
11. Determine the number of atomic structure centers: 0
12. Number of uncertain atomic structure centers: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
And do you feel whether it is necessary for you test the sample from the batch befor you use it,using Professional equipment ? the anwer is no doubt “yes” these equipments are needed.
For instance,
Through the kinds of special spectrum as follows ,can you want to restore theem as follows?
If you want to buy the chemical ,please inquiry us through E-mail: chem@pewax.com.cn
- standard HNMR picture of acetic acid-(2-butoxy-ethyl ester) CAS NO. 112-07-2 as follows
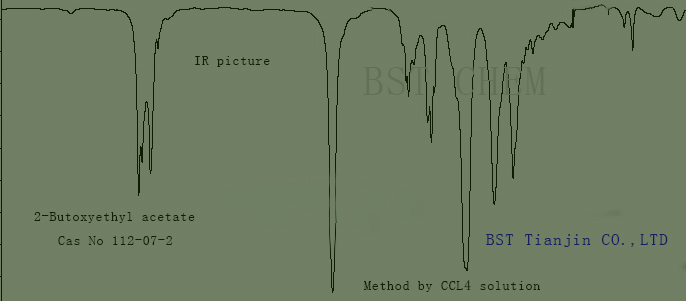
- standard IR piture of 1-Acetoxy-2-butoxyethane CAS NO. 112-07-2 as follows
if you want to get price, GC picture or other spectrumas, welcome e-mail us through info@pewax.com.cn
Will you hope the standard specification of Ethylene glycol mono-n-butyl ether acetate CAS NO. 112-07-2 in general?
The specification of Butoxyethanol acetate is as follows
Colorless and transparent liquid in appearance
Chromaticity (Pt Co) ≤ 10#
Content,% 99%
Acid value (calculated as HAc) ≤ 0.02
Relative density (20 ℃/20 ℃) 0.9400~0.9440
Moisture (wt),% ≤ 0.05

Of course,the most important thing is what are the usages of Diethylene glycol monobutyl ether acetate,do you
want to know?
If you want to buy the chemical ,please inquiry us through E-mail: chem@pewax.com.cn
Mainly used as an ink oil and baking glaze oil, especially suitable for high-end paints such as screen printing ink, car paint, television paint, refrigerator paint, aircraft paint, etc.
Other usages as below
Ethylene glycol butyl ether acetate, also known as BCA, is a very important industrial solvent. The molecular structure of BCA has both non polar parts and polar groups, thanks to which BCA has good solubility for both polar and non polar substances. In addition, BCA has the characteristics of good thermal stability, small viscosity changes, high foaming, and low corrosion. Therefore, it can be used as a coalescing agent for latex paint, as well as a solvent for metal and furniture painting. It can be used as a solvent for protective coatings, dyes, resins, leather, ink, and in the formulation of surface cleaning agents for metals, glass, and other surfaces. It is a widely used and high value-added fine chemical product.
if you have known other new usages,welcome e-mail us through info@pewax.com.cn
we can disucss each other on such new fields.
If you want to buy the chemical ,please inquiry us through E-mail: chem@pewax.com.cn
Ultimately, articles cited as follows, for your reference,we do not know if you can get some helps from them then? Related scientific Articles:
(1)CERAPP (Collaborative Estrogen Receptor Activity Prediction Project) is a large-scale modeling project demonstrate the efficacy of using predictive computational models trained on high-throughput screening data to evaluate thousands of chemicals for ER-related activity and prioritize them for further testing. CERAPP combined multiple models developed in collaboration with 17 groups........................
(2)
The Consumer product category classification is based on the Consumer Product Information Database ©2001-2021 by DeLima Associates.