Tetrahydropyran
If you need the products .Please send your inquiry to us through e-mail: cheminquiry@163.com
First of all, let us tell you what is the basic information of Tetrahydropyran.
Common Name Tetrahydropyran Boiling Point 88.0±0.0 °C at 760 mmHg
CAS Number 142-68-7 Molecular Weight 86.132
Density 0.9±0.1 g/cm3 Molecular Formula C5H10O
Melting Point −45 °C(lit.) Symbol dangerous
MSDS Document Flash Point -15.6±0.0 °C
Like many stuff, it has many synonyms as follows
2H-Tetrahydropyran
Peroxan
EINECS 205-552-8
1,5-EPOXYPENTANE
Etrahydropyran
Tetarhydropyran
4,5-dihydro-2H-pyran
tetrahydro-4H-pyran
tetrahydropyrane
OXACYCLOHEXANE
tetrahydro-2h-pyra
4-tetrahydropyran
2H-Tetrahydropyran
2H-Pyran, tetrahydro-
T6OTJ
Tetrahydro-2H-pyran
THP
Oxane
Pentamethylene Oxide
The chemical is very special,so we want to show some special properties as follows
Properties and Stability
Stable at room temperature and pressure. During storage, explosive peroxides can be formed, so reducing agents such as sodium bisulfite and stannous chloride are often added to suppress the formation of peroxides. Reacts fiercely with acidic substances. It is unstable when heating and pressurizing, and attention should be paid when using it.
Chemical properties: Tetrahydropyran, like aliphatic ethers, is chemically inactive and can generate peroxides under the action of oxygen in the air. Light has a promoting effect on the generation of peroxides and reacts with chlorine to obtain various chlorotetrahydropyrans. React with acyl chloride to form ω- Chloropentyl ester. React with ammonia, aliphatic amines, and aromatic amines to obtain piperidine or piperidine substituents.
Second, the Synthetic Route we will recommend is the most important for your reference?
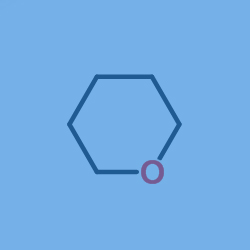
The synthesis line in the lab is the preparation of 1,5-pentanediol (starting material) and 1-butyl-3-methylimidazolium chloride (ionic liquid) with a total input of 0.3g was introduced into quartz capillary tubes in a ratio of 1:1.7 (weight ratio) (Inner diameter 2.5mm, length 20cm, volume 1ml, and seal it. When heated at 180 ° C for 12 hours, phase separation occurs in the capillary and tetrahydropyran forms in the upper layer. The recovery rate is 50%. According to 1H-NMR analysis, the purity of the recovered tetrahydropyran is 90% (no moisture detected: less than 0.1%).
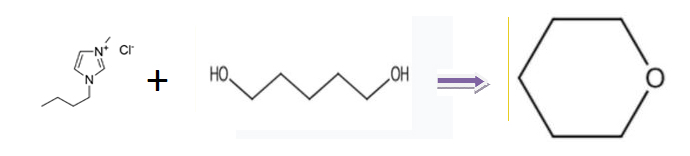
Other synthesis lines of OXACYCLOHEXANE as below
Third, what is the usage of Tetrahydropyran ?
Used for 1,5-Dichloropentane, which can be used to synthesize heptadine and heptadoic acid,
And used for the production of synthetic fibers.
It can also be used as a pharmaceutical intermediate
Besides Safety Information is also important when handling it
Dangerous goods markings F, Xi, Xn
Hazard category codes 11-36/37/38-9/19-52/53-22
Safety instructions 9-16-23-36-36-Chemicalbook61
Dangerous Goods Transport Number UN19933/PG2WGKGermany3TSCAYes
Hazard level 3
Packaging Category II
Customs code 2932110000
What is the appearance of Tetrahydropyran ?
Please see the picture below

Third,How can you identify if the chemical you have is right or not?
through conforming the a collection of illustrative plates as follows
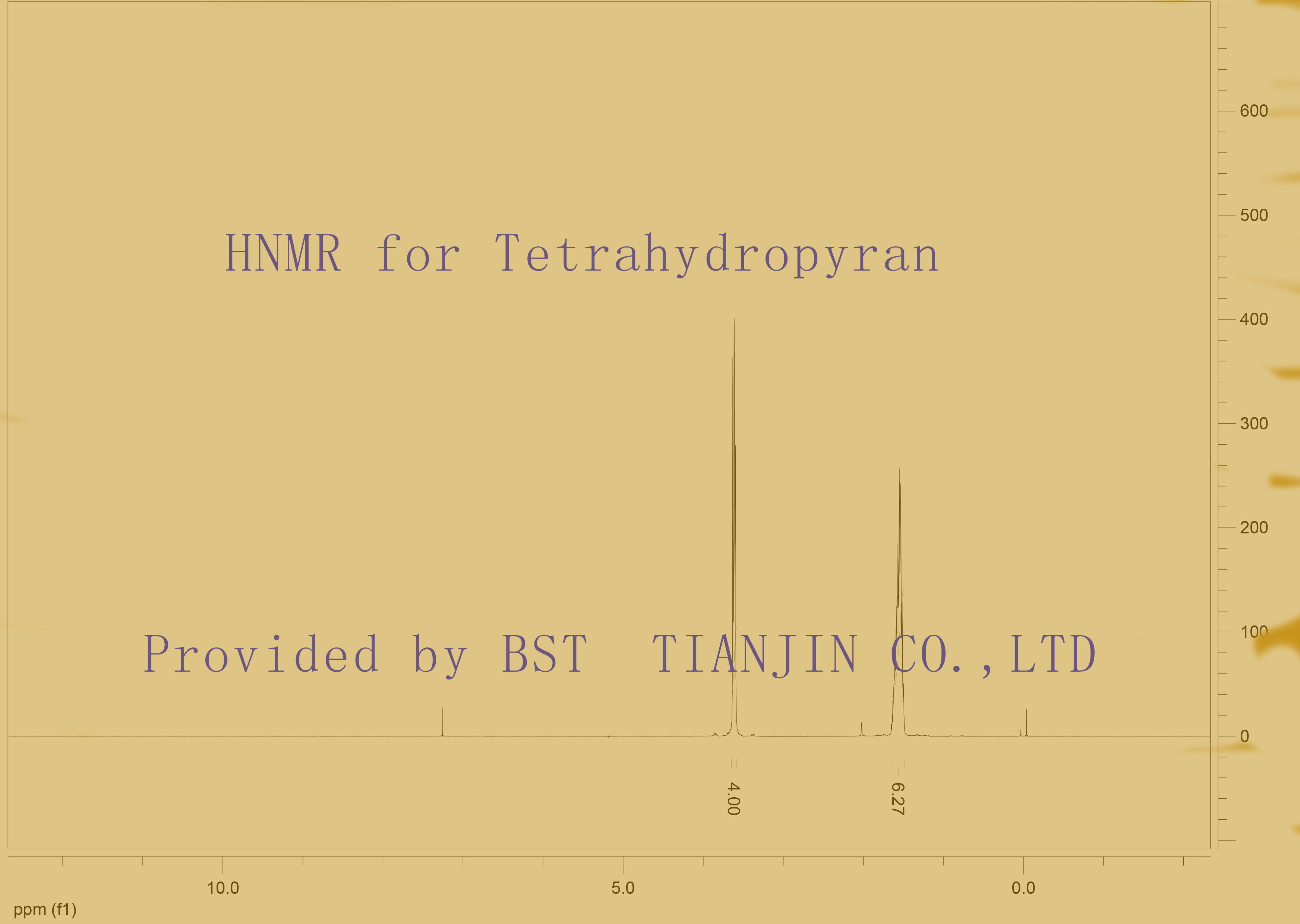
HNMR is below
IR picture of Tetrahydropyran as follows
Specificaiotn:
Appearance: colorless liquid
Purity:99% min by GC
IR and HNMR identify: conform
Water:0.5% max by KF
Article cited for your reference below
1.Compounds such as tetrahydropyran-2-one (67), with the keto function adjacent to the heteroatom, behave as cyclic esters, and are ring-opened by hydroxide ion.From: Comprehensive Heterocyclic Chemistry, 1984
2.the dangerous characters from pubchem


